Reference no: EM131311493
Assignment
Part 1
1. The pH scale is generally considered as a scale from 1 to 14. State a value of pH considered to be:
a. acidic
b. basic
c. neutral
2. The acid in our stomach is used to break down food proteins and kill bacteria and has what value pH?
3. Our blood pH is what value?
4. A solution in which pH does not change very much when small amounts of acid or base are added to it, is called a
5. Give 4 acids that are found as ingredients in food. Note that this means they are added to the food, not present naturally.
6. What is the major acid found in coffee?
7. What is the typical pH of coffee?
8. What is the reaction responsible for the browning of food that gives colors and flavors when foods are baked, roasted, or toasted?
9. What is the major antioxidant found in cocoa?
10. To what class of compounds does the antioxidant in the question 4 above belong, and how is it affected by the alkalization process?
11. What is the typical solution used to alkalize cocoa?
12. Why (for what purpose) is cocoa alkalized?
13. What is the typical pH of cocoa powder, and then what is the pH after alkalization?
14. The calorie (cal) is the standard energy unit listed on food nutrition labels. How many calories do we get from food containing:
1. One gram of fat
2. One gram of sugar
3. One gram of protein
15. Use the reaction below to answer the questions that follow.
CH4 + O2 → CO2 + H2O (g) + 802 kcal
a. Is energy absorbed or released in this reaction?
b. This reaction is which of the following?
a. Exothermic b. Endothermic c. Monothermic d. Geothermic
c. If you are holding the jar containing this reaction, will your hands get cold, warm, or not change temperature?
d. A hot pack when activated is an example of an exothermic reaction. Give another example of an exothermic reaction:
e. A cold pack when activated is an example of an endothermic reaction. Give another example of an endothermic reaction:
16. What is a catalyst?
Part 2
1. Which element is the most electronegative?
O
Si
Al
Pb
2. Which of the following would you expect to have the highest boiling point? Electronegativities: C = 2.5, H = 2.1, N = 3.0, 0 = 3.5
CH3CH2CH3
CH3CH2OH
CH3CH2OCH3
CH3CH2NH2
3. The reason you those your answer to question 2 above is that this molecule:
a. would have the lowest intermolecular forces.
b. would have no hydrogen-bonding to hinder its entry into the gas phase.
c. would have the least ionic character.
d. would have the greatest intermolecular forces.
e. would be held in the liquid phase more loosely than the others.
4. Which is more soluble in water?
a. sugar b. butter c. triglycerides
5. Cocoa butter would be most soluble in which of the following:
a water b. ethanol c. hexane d. salt water
6. Which of the following makes up proteins?
a. amino acids b. fatty acids c. nucleotides d. monosaccharidcs
7. Fats differ from sugars in which of the following ways? (Circle all that apply.)
a. Sugars are more water soluble than fats.
b. Fats have ester bonds on a glycerol backbone while sugars contain alcohol groups.
c. Fats are more polar than sugars.
d. Fats have amide bonds on a glyceraol backbone while sugars contain ester groups.
8. Circle the correct end to this statement. If no intermolecular forces existed, all substances would:
a. boil at a higher temperature.
b. be bound together.
c. be in a solid state.
d. be in the gas state.
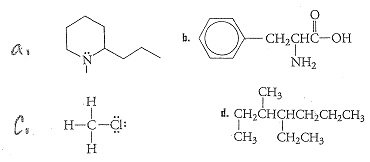
9. Which molecule below is the most polar?
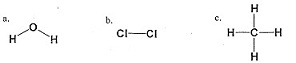
10. Which of the following molecules are polar? Each is drawn showing its actual three dimensional shape.
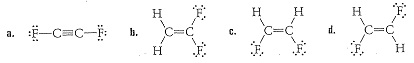
11. For the molecules shown below:
a. Indicate all polar bonds, and
b. List all the intermolecular forces each molecule experiences.