Reference no: EM131128171
1). Consider the following two reactions:
ADP + Pi -> ATP ΔGo' = 30.5 kJ/mole
Creatine + Pi -> Creatine phosphate ΔGo' = 43.1 kJ/mole
The enzyme creatine kinase can transfer the phosphate from ATP to creatine, thus generating creatine phosphate. Creatine phosphate can be used as a source of phosphate in muscle cells.
Write the equation for the reaction in which creatine phosphate is synthesized (as described above). What is the ΔGo' for this reaction? Would you consider this reaction to spontaneous or non-spontaneous at standard state?
In a recovering muscle cell, the following concentrations are observed:
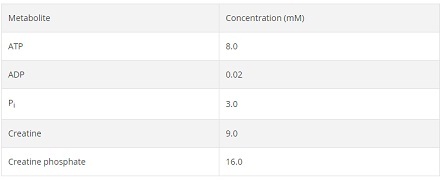
Calculate the ΔG' for creatine phosphate formation in these cells at 37oC. Is the reaction spontaneous in recovering muscle cells?
Is the ΔG' calculated in b) different from the ΔGo' that you calculated in a)? Why or why not?
During the first few seconds of heavy exercise in muscle cells, the ATP levels can drop to 0.8 mM and the ADP levels can increase to 0.2mM.What effect would this have on the spontaneity of the reaction in b)? What would you predict to happen to the reaction in a)?
What is the significance of this?
2) . You're a biochemist hired by NASA to study the results of a probe sent to Rhea, an earth sized planet several light years away. There is a large amount of excitement over Rhea as it contains several primitive life forms resembling plants that contain carbohydrates and proteins! It is currently hypothesized that these "plants" could provide a source of nutrients for any future manned missions to Rhea. The most recent "plant" data from the probe is listed below:
Carbohydrates:
Two disaccharides detected:
α-L-glucopyranose linked to β-L-fructofuranose in an α1-β2 arrangement.
β-L-galactopyranose linked to α-L-glucopyranose in an α1-4 arrangement.
Protein amino acid composition:
D-glycine 7.5%
D-alanine 7.2%
D-serine 5.7%
... More data in next transmission.
Armed with this, you dash into your supervisors' office!!!
What have you learned from this latest dataset from the probe? Why is it important?
3). Given the following reversible reaction:
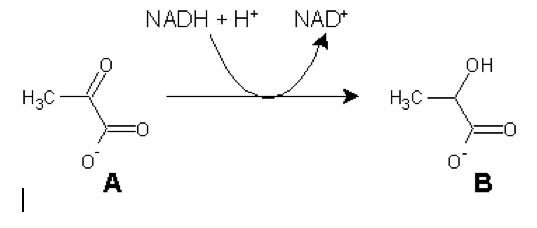
Identify A and B, as well as the enzyme that catalyzes this reaction (no peeking now... you can't peek during the exam).
What is the significance of this reaction? When would it occur (give an example)?
Suppose the ΔGo' of the above reaction is -25 kJ/mole. What is the highest ratio of products to reactants that would allow this reaction to go forward in the cell?
There are some very rare cases where the enzyme catalyzing the above reaction is mutated and inactive. These patients suffer from exertional myoglobinuria (the presence of myoglobin in the urine during exercise) and fatigue upon heavy exercise. Explain these symptoms (hint: Where is myoglobin normally found?).
|
Century national bank description
: Read the description of the Century Bank data set and complete the tasks described in the steps provided. Submit both a Microsoft Word and a Microsoft Excel document that contain the results of the tasks thatshow the completion of the tasks.
|
|
Journalize the adjusting entry on december 31
: Montana Mining Co. acquired mineral rights for $120,000,000. The mineral deposit is estimated at 200,000,000 tons. During the current year, 31,155,000 tons were mined and sold.
|
|
What are the two main groups into which cells are classified
: What are the two main groups into which cells are classified - Do the cells of bacteria have a nucleus?
|
|
What was the depreciation for the first year
: Assuming the equipment was sold at the end of the sixth year for $90,000; determine the gain or loss on the sale of the equipment.
|
|
What is the significance of the reaction
: Calculate the ΔG' for creatine phosphate formation in these cells at 37oC. Is the reaction spontaneous in recovering muscle cells?
|
|
Find the probability that tomorrow
: During a 69-year period, tornadoes killed 6755 people in the United States. Assume this rate holds true today and is constant throughout the year. Find the probability that tomorrow
|
|
Journalize the entry to record the sale
: Assuming the equipment was sold at the end of the second year for $200,000; determine the gain or loss on the sale of the equipment.
|
|
Determine the size of the milky way galaxy
: How did Harlow Shapley use the period-luminosity relationship of RR Lyrae variables to determine the size of the Milky Way galaxy
|
|
Tough economic times-the indiana legislature
: Because of tough economic times, the Indiana legislature is debating a bill that could have significant negative implications for public school funding. There are three possibilities for this bill:
|