Reference no: EM133284849
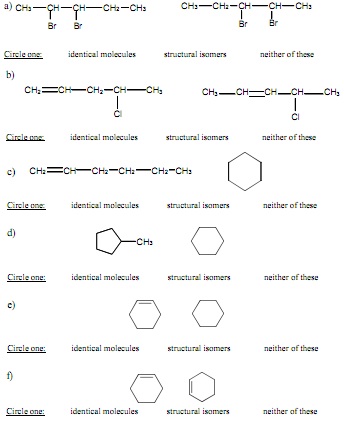
Structural Isomers of Alkanes
In each pair, determine whether the molecules are isomers, identical molecules, or neither of these.
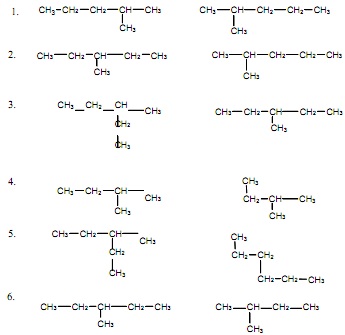
Naming Alkanes
Name the following molecules using IUPAC rules.
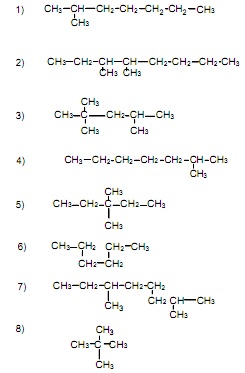
Alkane Structures
Draw a structure for each compound. Helpful Hint: Draw the carbon skeleton, then add in the appropriate number of hydrogens for each carbon, keeping in mind that each carbon in the molecule makes a total of four bonds. Also, the molecular formula for any alkane is CnH2n+2.
1. 2,5-dimethyloctane
2. 3-methyldecane
3. 2,4-dimethylheptane
4. 2,3,3-trimethyloctane
5. 4-ethylnonane
6. 2,2,4,5-tetramethyloctane
7. 2-methylbutane
8. 2-ethylpropane (what is better name for this compound?)
Naming Alkenes
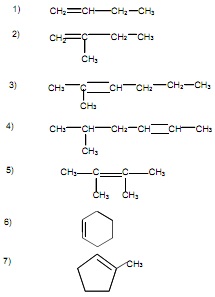
Alkene Structures
Draw a structure for each compound. Helpful Hint: Draw the carbon skeleton, then add in the appropriate number of hydrogens for each carbon, keeping in mind that each carbon in the molecule makes a total of four bonds.
1. 2,2-dimethyl-3-hexene
2. 4-ethyl-2-octene
3. 2-methylpropene
4. cyclobutene
5. 2,3-dimethyl-3-heptene
6. 1-ethylcyclopentene
7. 2,3,4-trimethyl-3-hexene
8. 1,1,2,2-tetramethylethene (what is better name for this compound?)
Structural Formulas and Isomers
1. What is the relationship between each pair of molecules?