Reference no: EM131846646
Experiment - Grignard Synthesis of Triphenylmethanol from benzophenone
Lab Manual: Macroscale and Microscale Organic experiments
Procedure: (Microscale procedure)
1. Weigh ~50 mg of Mg turnings and put it in the round bottom flask (long neck).
2. Add 0.5 mL of dry ether to the Mg using the syringe to measure the volume.
3. Cover the flask loosely with the septum.
4. Weigh ~330 mg of bromobenzene in a glass vial. Record the actual weight in the table of reactants and products above.
5. Add 0.7 mL of dry ether to the bromobenzene and mix the two liquids by gently shaking the vial.
6. Add ~0.1 mL of the bromobenzene solution to the flask containing the Mg.
7. Use the glass rod to crush the Mg on the bottom of the reaction tube. This exposes a fresh, non-oxidized surface on the metal that will react with the bromobenzene. The reaction has started when the solution becomes cloudy and bubbles start to form.
8. When the reaction starts, add the stir bar to the flask, cover it with a septum, stick a needle through the septum and stick the full syringe into the septum. Wrap a dampened paper towel around the neck of the flask. Clamp the flask onto a bar over a stir plate and stir the solution.
9. Fill the syringe with the bromobenzene solution.
10. SLOWLY ADD THE BROMOBENZENE SOLUTION over the next 10 to 20 minutes. The reaction should reflux gently during this period until you have no metal or very small quantity of the metal. You can place the flask in a water bath to keep to reaction from heating up.
11. While you are waiting for the reaction above to finish, prepare the benzophenone solution by weighing ~0.364 g of benzophenone in a glass vial.
12. Add 1 mL of dry ether to this vial and mix the contents until the benzophenone dissolves.
13. When the first reaction is complete, fill the syringe with the benzophenone solution and add it to the flask slowly, maintaining a gentle reflux. This reaction goes a bit faster than the previous one.
14. When a solid forms in the flask the reaction is over.
15. Cool the flask in an ice bath.
16. SLOWLY add 2.0 mL of 3M HCl to the flask and stir until the solid dissolves. The reaction is exothermic it will become hot if this is done too quickly.
17. Using a pipette, transfer the solution to a pointed bottom tube. Rinse the reaction tube with 1-2 mL of MTBE (methyl tert-butyl ether) and add this to the pointed bottom flask.
18. Use a glass pipette to remove the aqueous layer. Discard the aqueous layer.
19. Wash the ether layer with sat. NaCl solution, by adding 2-3 mL of NaCl solution, mixing the layers and removing and discarding the aqueous layer.
20. Dry the ether layer over MgSO4.
21. Evaporate the filtrate to get the product.
22. Add 2~3 mL of petroleum ether to your crude product and stir (or grind) the solid as you cool the mixture in an ice bath. This is trituration.
23. Remove the petroleum ether and dry your product on the bench.
24. Weigh your product and calculate the yield. Keep the pure product in your drawer for IR spectroscopy.
Result and Discussion
1. What compound did you make in the procedure 10?
2. What did you make in the procedure 13?
3. What is the purpose of adding 3M HCl to the reaction mixture?
4. What is the purpose of ether extraction?
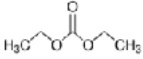
5. Triphenylmethanol can also be prepared by reacting ethyl benzoate with phenylmagnesium bromide and by reacting diethyl carbonate (below) with phenylmagnesium bromide. Write the mechanisms for these 2 reactions.
6. What weight of dry ice is required to react with 2 mmol of phenylmagnesium bromide? Would excess influence the yield in the reaction?
7. In the synthesis of benzoic acid benzene is often detected as a side product. How does this come about?