Reference no: EM131040796
Question 1-
a. What is the oxidation state of manganese in the complex, [MnF6]3-? Show how you arrived at your answer.
b. What is the d electron configuration of Mn in this complex?
c. Draw and label a crystal field energy level diagram for the octahedral complex, [MnF6]3-.
d. What is the crystal field stabilisation energy (CFSE) of Mn in [MnF6]3-?
e. Two ligands are replace by H2O to give [MnF4(H2O)2]-. Sketch and label the two geometrical isomers of this complex.
f. Two F ligands in [MnF6]3- are replaced by ethylenediamine (en). Sketch the resulting complex. You can represent en by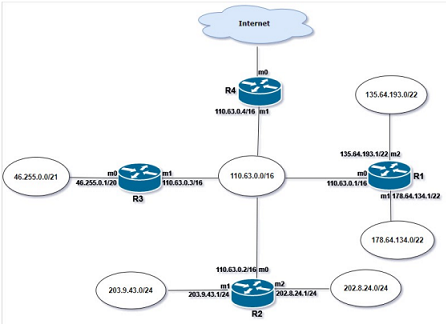 ?
?
g. Will the lines in the spectrum of the en-substituted complex arising from d↔d transitions be at higher or lower wavenumber than those of [MnF6]3-? Explain your answer.
h. Will the lines in the spectrum of the en-substituted complex arising from d↔d transitions be of higher or lower intensity than those of [MnF6]3-? Explain your answer.
Question 2-
Scheme 1 shows both the forward (synthetic) and backward (retrosynthetic) steps in a proposed synthesis of a pheromone of the cabbage looper moth, 8. You may not be familiar with the chemistry involved in every step in the scheme, but this should not affect your ability to answer the questions.
a. Explain in one or two sentences the significance of the two types of arrows in the scheme. (3 marks)
b. The following questions relate to the retrosynthetic analysis shown in Scheme 1.
1. For each step state whether it is an FGI, a C-C disconnection or a C-X disconnection.
2. Identify, by drawing the structures and labelling with a + or a - sign in the correct position, the positive and negative synthons A and B associated with step f.
3. Identify a reagent, 2, that corresponds to the electrophilic synthon at step 1.
4. Suggest a suitable reagent (C) for step 4 and a suitable reagent (D) for step 5. Briefly explain why your chosen reagent is suitable.
Question 3-
The target compound 9 can be synthesized from each of the following starting materials, 10, 11 and 12 by using suitable organometallic reagents.
For each of the three starting materials, specify the required organometallic reagent and reaction conditions (including work up if necessary) to complete the synthesis. (Hint: you might find it helpful to generate the structures of the target products from their names using Accelerysdraw or other drawing software).
- 9 is pentan-3-one (3-pentanone)
- 10 is propanenitrile (ethyl cyanide)
- 11 is propanoic acid (propionic acid)
- 12 is propanoyl bromide
Question 4-
We suggest you answer parts (a) and (b) by sketching the answers on paper and pasting them into your TMA as separate graphics.
a. Draw the Lewis structure for SeF4.
b. Determine the predominant product for reaction (1).
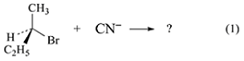
Draw out the mechanism for this reaction, paying attention to the stereochemistry.
c. Compound A has the empirical formula C8H14O. The off resonance 13C NMR data for compound A is given in Table 1, and the infrared spectrum is given in Figure 1.
Table 1: 13C NMR data for Compound A
|
Peak shift: δ/p.p.m.
|
Multiplicity
|
|
11.5
|
Quartet
|
|
21.8
|
Triplet
|
|
47.2
|
Doublet
|
|
64.5
|
Doublet
|
|
73.6
|
Doublet
|
|
84.3
|
Singlet
|

Figure 1 Infrared spectrum of compound A
i. Determine the number of double bond equivalents, if any, for compound A. (Give brief reasoning how you have arrived at your answer.)
ii. From the infrared spectrum of compound A (Figure 1) use the correlation charts in the S215 Data Book to assign the most likely type of absorption mode of the marked bands in the spectrum.
iii. Use the 13C NMR data provided in Table 1 to explain what information this gives about the nature of the carbon atoms in compound A.
iv. Using your answers given for parts (ii) and (iii) suggest and draw the structure of compound A.