Reference no: EM131214268
1.) Be sure to answer all parts. Enter your answers in scientific notation.
How many carbon atoms are contained in each of the following number of moles:
(a) 1.80 mol;
(b) 6.60 mol?
Report answer using three significant figures.
2.) How many molecules are contained in each of the following number of moles?
Report your answer using two significant figures.
a. 8.2 mol of penicillin molecules
b. 0.78 mol of NH3 molecules
3.) How many molecules are contained in each of the following number of moles?
Report your answer using correct significant figures.
a. 0.27 mol of sugar molecules
b. 52.8 mol of acetaminophen molecules
4.) L-Dopa is a drug used to treat Parkinson's disease.
(Write your molecular formula in the following format: CwHxNyOz)
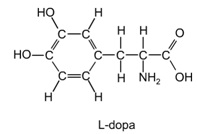
a. What is the molecular formula of L-dopa?
b. What is the molecular weight of L-dopa?
c. What is the molar mass of L-dopa?
5. How many grams are contained in 6.20 mol of each compound?
Do not convert these answers to scientific notation.
a. HCl
b. Na2SO4
6.) How many grams are contained in 6.50 mol of each compound? Do not convert these answers to scientific notation.
a. C2H2
b. Al(OH)3
7.) How many moles are contained in each number of grams of table sugar
(C12H22O11,molar mass 342.3 g/mol)? Enter your answer in scientific notation.
a. 0.320 g
b. 7.50 g
8.) Which has the greater mass: 0.24 mol of aspirin or 45.0 g of aspirin (C9H8O4)?
45.0 g of aspirin
0.24 mol of aspirin
9.)What is the mass in grams of 6.70 ×1020molecules of the pain reliever ibuprofen (C13H18O2, molar mass 206.3 g/mol)? Answer shouldbe provided in scientific notation.
|
Calculate karl pearsons coefficient or correlation
: Calculate Karl Pearson's Coefficient or correlation for the following data relating to overhead expenses and cost of production - Calculate Product moment Coefficient of correlation for the data.
|
|
Find the expected value
: (a) Find the expected value (in dollars) of the amount won by one entry. (b) Find the expected value (in dollars) if the cost of entering this sweepstakes is the cost of a postage stamp (34 cents)
|
|
Design of a smart application to solve business need
: The purpose of this assignment is to prepare a written report that will present a detail plan and design of a smart application to solve business need stated in a business case. In this assignment you are working in a group of 3-4 students but you..
|
|
Expected winnings far a ticket buyer
: If each ticket costs $3 and 8600 tickets are sold, what are the expected winnings far a ticket buyer? Express to at least three decimal place accuracy in dollar form (as opposed to cents).
|
|
What is the molecular formula of l-dopa
: What is the molecular formula of L-dopa? How many molecules are contained in each of the following number of moles? How many moles are contained in each number of grams of table sugar.
|
|
Expected winnings for a single ticket buyer
: What are the expected winnings for a single ticket buyer? Express to at least three decimal place accuracy in dollar form (as opposed to cents).
|
|
Playing dungeons and dragons with a group
: Phuc is playing Dungeons and Dragons with a group of three friends. To heal an injured friend's character, Phuc must roll one die and roll a number higher than that friend's damage level.
|
|
Find y correct to three significant figures
: find y correct to three significant figures.-- Find r in V=(4/3)Πr2, where V=311, Π = 3.14 -- Find the ratio of y-x:2y.
|
|
Experiment is conducted as follows
: 1. An experiment is conducted as follows: A die is rolled and the number of spots is noted. If the number of spots is 5 or fewer the experiment is over. If a 6 is rolled, then a coin is tossed and we note if it is H or T.
|