Reference no: EM131490637
Assignment
Question 1
The initial rates for a reaction A and B were studied using the following initial concentrations in mol/L.
Expt 1. [A]=0.300, [B]=0.400, rate=2.80x10-4 M/s
Expt 2. [A]=1.04, [B]=0.400, rate=2.80x10-4 M/s
Expt 3. [A]=0.695, [B]=1.63, rate=4.65x10-3 M/s
What is the order of reaction with respect to B?
a. 1
b. 0
c. insufficient information
d. 2
Question 2
Suppose the rate law for a reaction is
rate = k[X]1[Y]2
and the rate of the reaction is 0.820 M/min when [X] = 0.697 M. All other factors being equal, if [X] = 1.96 M, the rate of the reaction would be
a. 2.54 M/min
b. 2.31 M/min
Question 3
The concentration of reactant A was monitored over time and the following data were obtained:
t=0, [A]=4.53x10-3 M
t=292 s, [A]=2.26x10-3 M
t=584 s, [A]=1.13x10-3 M
Which of the following rate laws is consistent with the data?
a. rate = (7.56x10-1 M-1 s-1) [A]2
b. rate = (2.37x10-3 s-1) [A]
c. rate = (7.56x10-1 s-1) [A]
d. rate = (2.37x10-3 M-1 s-1) [A]2
Question 4
The internuclear distance between atoms "A" and "B" in diatomic molecule AB is 1.36 Angstrom. If the molecule has a dipole moment of 3.23 Debye, calculate the percent ionic character.
a. 56.8%
b. 55.7%
c. 49.4%
Question 5
In the following question, treat bonds between atoms with electronegativity difference less than 0.5 (on the Pauling scale) as nonpolar.
The molecule H2S
a. has no polar bonds and is nonpolar
b. has no polar bonds but is polar
c. has polar bonds and is polar
d. has polar bonds but is nonpolar
Question 6
What type of intermolecular forces is due to induced (temporary) dipole moments in molecules?
a. Hydrogen bonding
b. London dispersion forces
c. Dipole-dipole interaction
Question 7
The structure of BeF2 can be considered as of the type AXmEn, where one central atom (A) is surrounded by m terminal atoms (X) and has n lone pairs. In this particular case, the central atom
a...
a. has an octet (which includes two lone pairs)
b. has an octet (which includes one lone pair)
c. has less than an octet (two pairs short)
d. has an octet with no lone pair
e. has less than an octet (one pair short)
Question 8
Which of the following are resonance structures?
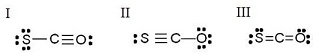
a. I and II only
b. I and III only
c. I, II, and III
d. II and III only
Question 9
A pure sample of liquid X has a normal boiling point of 63.5oC and a boiling point elevation constant of 4.93 oC mol-1 kg. When liquid X is mixed with nonvolatile solute particles, the boiling point of the resulting mixture was found to be 68.9oC. Calculate the molality of solute particles in the solution.
a. 1.04 mol/kg
b. 1.10 mol/kg
c. 14.0 mol/kg
d. 12.9 mol/kg
Question 10
A solution is prepared by mixing 37.2 mL of 0.389 M potassium phosphate(aq) and 43.0 mL of 0.490 M potassium sulfate(aq), then adding enough water to bring the total volume to 200.0 mL. Calculate the molarity of K+ in the resulting solution.
a. 4.28x10-1 M
b. 3.64x10-1 M
c. 2.75 M
d. 2.34 M
Question 11
A pure sample of liquid X has a normal freezing point of 11.9oC and a freezing point depression constant of 5.24 oC mol-1 kg. When 575 g of liquid X is mixed with 0.650 mol nonvolatile solute particles, the freezing point of the resulting mixture will be...
a. 18.7 oC
b. 5.98 oC
c. 17.8 oC
d. 5.68 oC
Question 12
What is the molar concentration of sodium ions in a 0.330M NaNO3(aq) solution?
a. 2.89 M Na+
b. 3.03 M Na+
c. 0.330 M Na+
d. 3.46x10-1 M Na+
Question 13
Which of the following salts yields an acidic solution in water?
a. LiF
b. AlCl3
c. NaNO3
d. NaI
e. KBr
Question 14
An insoluble salt made up of M2+ and X- ions has a Ksp of 8.50x10-11. Calculate the solubility.
a. 1.33x10-3 mol/L
b. 6.39x10-3 mol/L
c. 2.77x10-4 mol/L
d. 9.22x10-6 mol/L
Question 15
Which of the following is the conjugate acid of HCO3-?
a. H3CO3+
b. CO32-
c. HCO32-
d. H2CO3
e. HCO3-
Question 16
The pH of a 9.05x10-2 M solution of B(aq) is 11.706. Calculate the Kb.
a. 3.02x10-4
b. 2.85x10-4
c. 2.57x10-4
Question 17
Which of the following is a possible pH of a 2.99x10-3 M solution of B(aq) if B is a weak base?
a. 11.476
b. 9.257
c. 2.524
Question 18
Predict if a precipitate of M2X would be formed in a solution that is 2.00x10-5M in M+ and 3.53x103M in X2-. The Ksp of M2X is 1.66x10-6.
a. No, a precipitate will not be formed.
b. Yes, a precipitate will be formed.
Question 19
Which of the following describes the effect of removing products from a reaction mixture at equilibrium? (Q is the reaction quotient, Keq is the equilibrium constant)
a. increase in Q; reaction shifts to the right until Q=Keq again
b. change in Keq; reaction shifts to the left or right until Q=Keq again
c. decrease in Q; reaction shifts to the right until Q=Keq again
d. increase in Q; reaction shifts to the left until Q=Keq again
e. decrease in Q; reaction shifts to the left until Q=Keq again
Question 20
Consider the reaction: A(g) + B(g) = 2C(g), Kc = 30.0.
Suppose an 4.70-L mixture initially contains 3.56x10-3 mol A, 3.56x10-3 mol B, and no C. Calculate the equilibrium concentration of
a.
a. 2.13x10-4 mol/L
b. 1.11x10-3 mol/L
c. 2.03x10-4 mol/L
Question 21
How are the equilibrium constants Kp and Kc related for the following reaction:
2 NO2(g) → 2 NO(g) + O2(g)
a. Kc = (Kp)(RT)2
b. Kp = Kc
c. Kc = (Kp)(RT)
d. Kp = (Kc)(RT)
e. Kp = (Kc)(RT)2
Question 22
Consider the following fictitious reaction at equilibrium:
A(g) + B(g) ↔ C(g) ΔH = -80 kJ
Which of the following stresses would shift the equilibrium to the left?
a. decreasing the volume by increasing the pressure at constant temperature
b. adding more A
c. adding more C
d. decreasing the temperature
e. adding a catalyst
Question 23
Given the following standard reduction potentials:
Cu|Cu2+, 0.34 V
Ag|Ag+, 0.80 V
Based on the given information, under standard conditions, True or False:
Cu2+ is a better reducing agent than Ag+.
a. False
b. True
Question 24
State whether O is oxidized or reduced in
nitric oxide → nitrogen dioxide
a. neither
b. oxidized
c. reduced