Reference no: EM133110020
PBS-ANC-311 Analytical Chemistry
Question 1:
a) What are the three parts of quality assurance?
b) How can you validate precision and accuracy?
c) Distinguish raw data, treated data, and results.
d) What is the difference between a false positive and a false negative?
Question 2: a) From previous measurements of a low concentration of analyte, the signal detection limit was estimated to be in the low nanoampere range. Signals from seven replicate samples with a concentration about three times the detection limit were 5.0, 5.0, 5.2, 4.2, 4.6, 6.0, and 4.9 nA. Reagent blanks gave values of 1.4, 2.2, 1.7, 0.9, 0.4, 1.5, and 0.7 nA. The slope of the calibration curve for higher concentrations is m = 0.229 nA/mM.
i. Find the signal detection limit and the minimum detectable concentration.
ii. What is the concentration of analyte in a sample that gave a signal of 7.0 Na?
b) Olympic athletes are tested to see if they are using illegal performance-enhancing drugs. Suppose that urine samples are taken and analyzed and the rate of false positive results is 1%. Suppose also that it is too expensive to refine the method to reduce the rate of false positive results. We do not want to accuse innocent people of using illegal drugs. What can you do to reduce the rate of false accusations even though the test always has a false positive rate of 1%?
c) Europium is a lanthanide element found at parts per billion levels in natural waters. It can be measured from the intensity of orange light emitted when a solution is illuminated with ultraviolet radiation. Certain organic compounds that bind Eu (III) are required to enhance the emission. The figure below shows standard addition experiments in which 10.00 mL of sample and 20.00 mL containing a large excess of organic additive were placed in 50-mL volumetric flasks. Then Eu (III) standards (0, 5.00, 10.00, or 15.00 mL) were added and the flasks were diluted to 50.0 mL with H2O. Standards added to tap water contained 0.152 ng/mL (ppb) of Eu (III), but those added to pond water were 100 times more concentrated (15.2 ng/mL). Calculate the concentration of Eu (III) (ng/mL) in pond water and tap water.
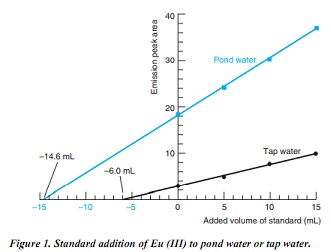
d) For tap water, emission peak area increases by 4.61 units when 10.00 mL of 0.152 ng/mL standard are added. This response is 4.61units/1.52 ng 5 3.03 units per ng of Eu (III). For pond water, the response is 12.5 units when 10.00 mL of 15.2 ng/mL standard are added, or 0.082 2 units per ng.
i. How would you explain these observations?
ii. Why was standard addition necessary for this analysis?
Question 3:
a) An iron ore was analyzed by dissolving a 1.1324-g sample in concentrated HCl. The resulting solution was diluted with water, and the iron (III) was precipitated as the hydrous oxide Fe2O3.xH2O by the addition of NH3. After filtration and washing, the residue was ignited at a high temperature to give 0.5394 g of pure Fe2O3 (159.69 g/mol). Calculate;
i. the % Fe (55.847 g/mol)
ii. the % Fe3O4 (231.54 g/mol) in the sample.
b) A 0.2121-g sample of an organic compound was burned in a stream of oxygen, and the CO2 produced was collected in a solution of barium hydroxide. Calculate the percentage of carbon in the sample if 0.6006 g of BaCO3 was formed.
c) The efficiency of a particular catalyst is highly dependent on its zirconium content. The starting material for this preparation is received in batches that assay between 68% and 84% ZrCl4. Routine analysis based on precipitation of AgCl is feasible, it having been established that there are no sources of chloride ion other than the ZrCl4 in the sample.
i. What sample mass should be taken to ensure an AgCl precipitate that weighs at least 0.400 g?
ii. If this sample mass is used, what is the maximum mass of AgCl that can be expected in this analysis?
iii. To simplify calculations, what sample mass should be taken to have the percentage of ZrCl4 exceed the mass of AgCl produced by a factor of 100?
Question 4:
a) A 6.881-g sample containing magnesium chloride and sodium chloride was dissolved in sufficient water to give 500 mL of solution. Analysis for the chloride content of a 50.0-mL aliquot resulted in the formation of 0.5923 g of AgCl. The magnesium in a second 50.0-mL aliquot was precipitated as MgNH4PO4; on ignition, 0.1796 g of Mg2P2O7 was found. Calculate the percentage of MgCl2.6H2O and of NaCl in the sample.
b) An alloy of chromel containing Ni, Fe, and Cr was analyzed by a complexation titration using EDTA as the titrant. A 0.7176-g sample of the alloy was dissolved in HNO3 and diluted to 250 mL in a volumetric flask. A 50.00-mL aliquot of the sample, treated with pyrophosphate to mask the Fe and Cr, required 26.14 mL of 0.05831 M EDTA to reach the murexide end point. A second 50.00-mL aliquot was treated with hexamethylenetetramine to mask the Cr. Titrating with 0.05831 M EDTA required 35.43 mL to reach the murexide end point. Finally, a third 50.00-mL aliquot was treated with 50.00 mL of 0.05831 M EDTA, and back titrated to the murexide end point with 6.21 mL of 0.06316 M Cu2+. Calculate the weight percent of Ni, Fe, and Cr in the alloy.
c) A 0.5000-g sample containing NaHCO3, Na2CO3, and H2O was dissolved and diluted to 250.0 mL. A 25.00-mL aliquot was then boiled with 50.00 mL of 0.01255 M HCl. After cooling, the excess acid in the solution required 2.34 mL of 0.01063 M NaOH when titrated to a phenolphthalein end point. A second 25.00-mL aliquot was then treated with an excess of BaCl2 and 25.00 mL of the base. All the carbonate precipitated, and 7.63 mL of the HCl was required to titrate the excess base. Determine the composition of the mixture.
d) A 1.219-g sample containing (NH4)2SO4, NH4NO3, and nonreactive substances was diluted to 200 mL in a volumetric flask. A 50.00-mL aliquot was made basic with strong alkali, and the liberated NH3 was distilled into 30.00 mL of 0.08421 M HCl. The excess HCl required 10.17 mL of 0.08802 M NaOH for neutralization. A 25.00-mL aliquot of the sample was made alkaline after the addition of Devarda's alloy, and the NO3-1 was reduced to NH3. The NH3 from both NH+and NO-1 was then distilled into 30.00 mL of the standard acid and back- titrated with 14.16 mL of the base. Calculate the percentage of (NH4)2SO4 and NH4NO3 in the sample.
Attachment:- Analytical Chemistry.rar