Reference no: EM131516164
Part A -
I. Half-life(t½) & amount of radioisotope that remains [At = A0(1/2)n], where n = # of t½.
1. A radon isotope is an alpha-emitter with a half-life of 3.8 days. What is the mass of Rn-222 that remains after 15.2 days in a sample of the gas containing 4.38g of the radioisotope?
2. What is the fraction of an isotope of Co-60 that remains after 3 half-lives? What is this in percent?
3. Radium-224 undergoes alpha emission with t½ = 3.64 days. Starting with a 5.00 g sample, what % remains radioactive after 7.00 days?
4. Strontium-90 decays through beta emission. After 58 years, it is found that 25% of the original sample remains. What is the half-life of strontium-90?
5. Calculate the time required for three-fourths of a sample of cesium-138 to decay given that its half-life is 32.2 minutes.
6. Calculate the half-life of cesium-135 if seven-eighths of a sample decays in 6.0 x 106 years.
7. How many milligrams decay of a 15.000mg sample of radium-224 after 6396y if the half-life of the radioisotope is 1599y?
8. The half-life of tritium is 12.3y. How long will it take for seven-eighths of the sample to decay?
9. The half-life of radium-224 is 3.66d. What was the original mass of the radioisotope if .0500g remains after 7.32d?
II. Solve the following nuclear reactions problems. Show all of your work!
1. The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 60Ni nucleus? (The mass of a nickel-60 nucleus is 59.9308 amu.)
2. The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 6027Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
3. The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy (in J) of 6027Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
4. The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the binding energy per nucleon (in J) of a 6027Co nucleus? (The mass of a cobalt-60 nucleus is 59.9338 amu.)
5. The mass of a proton is 1.673 x 10-24 g. The mass of a neutron is 1.675 x 10-24 g. The mass of the nucleus of an 56Fe atom is 9.289 x 10-23 g. What is the nuclear binding energy (in J) for 56Fe?
6. When two atoms of 2H are fused to form one atom of 4He, the total energy evolved is 3.38 x 10-12 J. What is the total change in mass (in kg) for this reaction?
7. Carbon-11 decays by positron emission: Write the balanced nuclear equation for this reaction.
8. The decay above occurs with a release of 2.87 x 1011 J per mole of carbon-11. When 4.00 g of carbon-11 undergoes radioactive decay, ______ g of mass is converted to energy.
9. How much energy (in J) is produced when 0.082 g of matter is converted to energy?
Part B -
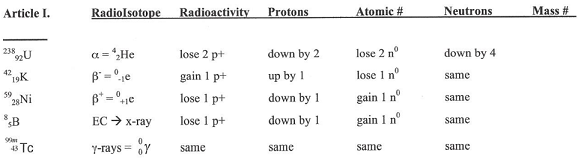
*Gamma radiation usually accompanies any of the above radioactive decay processes.
**All nuclei with atomic number greater than 83 are radioactive and therefore undergo radioactive decay.
***Radioisotopes with longer half-lives are more stable than radioisotopes with shorter half-lives.
A. Explain what takes place inside the nucleus during each of the following, use a particle-decay equation (β-, β+, EC) to indicate the nuclear change(s):
1. alpha-decay:
2. beta-decay:
3. positron-emission:
4. electron-capture:
B. Write balanced nuclear equations for the following nuclear reactions.
1. The alpha-decay of uranium-235.
2. The beta-decay of iodine-131.
3. The positron emission of phosphorus-30.
4. The electron capture and x-ray emission in zirconium-84.
C. Nuclear Bombardment (alpha, beta, neutrons, or protons), FISSION, and element synthesis. Write a nuclear equation for each reaction.
1. When nitrogen-14 is bombarded with alpha-particles oxygen-17 and a proton are produced.
2. If aluminum-27 is bombarded with a neutron, magnesium-27 and a proton result.
3. Uranium-238, when bombarded with deuterium, forms the unstable nuclide neptunium-238 and neutrons.
4. One way that a uranium-235 nucleus undergoes fission is that it absorbs a slow neutron and produces barium-139 and kryption-94.
D. Nuclear Bombardment (alpha, beta, neutrons, or protons), FUSION, and element synthesis. Write a nuclear equation for each reaction.
1. Two tritium atoms undergo nuclear fusion and produce helium-4 and neutrons.
2. The nuclear fusion reaction that occurs in the sun fuses one deuterium nuclide with one tritium nuclide producing one helium-4 nuclide and a neutron.
3. Uranium-238 is fused with neon-22 to produce what new element and a neutron.
4. Lead-210 and nickel-59 undergo fusion in order to synthesize element 110 with a mass of 269.
E. Write the balanced nuclear equations for the following nuclear reactions.
1. Alpha followed by gamma emissions of radon-222
2. Beta emission of bismith-215
3. Sulfur-35 will decay by ________
Write the nuclear equation.
4. Sodium-24 will decay by ________
Write the nuclear equation.
5. Cobalt-60 will decay by ________
Write the nuclear equation.
6. Electron capture of fluorine-17
7. Bombardment of calcium-41 with a beta-particle
8. Bombardment of curium-246 with carbon-13 to produce nobelium-254
9. Bombardment of lithium-6 with to produce alpha-particles & tritium
10. Bombardment of boron-10 with α-particles to produce carbon-13.