Reference no: EM133289860
Gen Organic and Biochemistry
Question 1
A magnesium ion, Mg2+, has
- 12 protons and 14 electrons.
- 24 protons and 26 electrons.
- 24 protons and 22 electrons
- 12 protons and 10 electrons.
- 12 protons and 13 electrons.
Question 2
The correct name for NH4NO2 is
A) hydrogen nitrogen oxide.
B) hydrogen nitrate
C) ammonium nitrite
D) ammonium nitrate
E) ammonia nitrogen dioxide
Question 3 What is the formula for the ionic compound formed by magnesium and iodine?
Question 4 Which one of the following elements is most likely to form a 2- ion?
A) strontium
B) iodine
C) selenium
D) scandium
E) silicon
Question 5 The correct name for KHCO3 is
A) calcium hydrogen carbon trioxide
B) potassium carbonate
C) calcium bicarbonate
D) potassium hydrogen carbonate
E) calcium carbonate
Question 6 What is a cation?
- A cation is defined as an atom that has gained electrons and forms a positive charge.
- A cation is defined as an atom that has lost electrons and forms a positive charge
- A cation is defined as an atom that has gained electrons and forms a negative charge
- A cation is defined as an atom that has lost electrons and forms a negative charge.
Question 7 What is the formula for the binary compound formed by potassium and nitrogen?
A) NK2
B) KN
C) K2N
D) NK3
E) K3N
Question 8 Which of the following pairs of elements would be most likely to form an ionic compound?
A) C and S
B) Al and K
C) Al and Mg
D) Cl and I
E) Cl and Mg
Question 9 The Lewis dot symbol for the S2- ion is
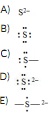
Question 10 Which of the following is the formula for hydrobromic acid?
A) HBr
B) HBrO3
C) KBr
D) HBrO2
E) HBrO
Question 11 An aluminum ion, Al3+, has:
A) 13 protons and 13 electrons
B) 16 protons and 13 electrons
C) 10 protons and 13 electrons
D) 27 protons and 24 electrons
E) 13 protons and 10 electrons
Question 12 The chemical formula for iron(II) nitrate is:
Fe2(NO3)3
Fe(NO2)2
Fe(NO3)2
Fe2N3
F(NO2)2
Question 13 Which of the following Lewis structures is incorrect?
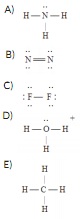
Question 14 The Lewis dot symbol for the a lead atom is
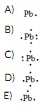
Question 15 The chemical name for ClO3- is chlorate ion. Therefore, the name of HClO3 is
A) chlorous acid.
B) hydrochloric acid.
C) chloroform.
D) chloric acid.
E) hydrogen trioxychloride.
Question 16
How many protons and electrons are present in one bromide ion?
80 p, 81 e
35 p, 34 e
80 p, 34 e
35 p, 36 e
35 p, 35 e
Question 17 Given that the ion ClO3- is named chlorate, what is the ion ClO4- named?
A) hypochlorite
B) perchlorite
C) chloride
D) perchlorate
E) chlorite
Question 18 Which pair of elements would be most likely to form an ionic compound?
Al and Rb C and S P and Br F and Al
Zn and K
Question 19 Predict the formula for the binary compound formed between barium and phosphorus.
A) Ba3P2
B) Ba2P3
C) Ba2P
D) BaP
E) BaP2
Question 20
Name the binary compound formed between barium and phosphorus.
A) barium diphosphate
B) barium phosphorus
C) barium triphosphide
D) barium(II) phosphate
E) barium phosphide
Question 21
The formula for sodium sulfide is
A) NaS2
B) SeS
C) Na2S
D) K2S
E) NaS
Question 22
What is the formula for the ionic compound formed by calcium and selenium?
A) Ca2Se
B) Ca3Se
C) CaSe2
D) CaSe
E) CaSe3
Question 23
A gallium ion, Ga3+, has:
A) 16 protons and 31 electrons
B) 31 protons and 28 electrons
C) 27 protons and 24 electrons
D) 31 protons and 31 electrons
E) 70 protons and 31 electrons
Question 24 An anion is defined as
A) an atom or group of atoms with a net positive charge.
B) a stable atom.
C) a group of stable atoms.
D) a charged atom or group of atoms with a net negative charge.
Question 25
The electron dot structure for AsCl3 shows
A) one single bond, two double bonds, and 8 lone pairs.
B) a total of 84 electron dots.
C) three single bonds and one lone pair.
D) two single bonds, one double bond, and 9 lone pairs.
E) three single bonds and 10 lone pairs.
Question 26
The number of lone electron pairs in the N2 molecule is ___.
A) 4
B) 5
C) 3
D) 2
E) 1
Question 27 An oxide ion, O2-, has:
A) 8 protons and 10 electrons
B) 10 protons and 7 electrons
C) 8 protons and 9 electrons
D) 8 protons and 7 electrons
E) 10 protons and 8 electrons
Question 28
Which is the correct formula for lead(IV) chloride?
PbCl3
Pb4Cl
PbCl4
PbCl2
Pb2Cl4
Question 29
Which is the correct formula for copper(II) phosphate?
A) Cu2PO4
B) Cu3(PO4)2
C) Cu(PO4)2
D) Cu(PO3)2
E) Cu2PO3
Question 30
The formula for calcium phosphite is
A) Ca3P2
B) Ca3(PO3)2
C) Ca2(PO4)3
D) CaPO4
E) Ca3(PO4)2
Question 31
The formula for potassium sulfide is
A) KS2
B) K2S
C) PS
D) P2S
E) NaS
Question 32 What is the formula for the ionic compound formed by calcium ions and nitrite ions?
A) Ca3N2
B) Ca(NO3)2
C) Ca2NO2
D) Ca(NO2)2
E) Ca2NO3
Question 33
Which of the following elements is chemically similar to magnesium?
A) calcium
B) nickel
C) iron
D) potassium
E) sulfur
Question 34 The Lewis dot symbol for the calcium ion is
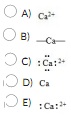
Question 35
A nitride ion has:
- 7 protons and 10 electrons
- 10 protons and 7 electrons
- 10 protons and 13 electrons
- 7 protons and 7 electrons
- 12 protons and 15 electrons
Question 36 The correct name for NH4NO3 is
A) hydrogen nitrogen oxide.
B) ammonium nitrate
C) ammonium nitrogen trioxide
D) ammonia nitrogen oxide
E) hydrogen nitrate
Question 37
The Stock system name for CrO3 is:
A) chromium(VI) oxide
B) chromium(II) oxide
C) chromium(III) oxide
D) chromium(III) trioxide
E) chromium oxide
Question 38 Which of the following elements is chemically similar to oxygen?
A) nickel
B) iron
C) sulfur
D) calcium
E) sodium
Question 39 Consistent with vanadium being a transition metal, the name for VSO4 should be
vanadium (II) sulfate
vanadium (IV) sulfite.
vanadium sulfur tetraoxide. vanadium (IV) sulfate.
vanadium sulfide.
Question 40
The total number of bonding electrons in a molecule of formaldehyde (H2CO) is
A) 6
B) 3
C) 18
D) 4
E) 8
Question 41 The formula for calcium phosphate is
A) Ca2(PO4)3
B) Ca3(PO4)2
C) Ca3P2
D) CaPO4
E) Ca3(PO3)2
Question 42 The Stock system name for Mn2O7 is
A) dimanganese heptaoxide
B) magnesium oxide
C) manganese(III) oxide
D) manganese(II) oxide
E) manganese(VII) oxide
Question 43 What is the formula for the ionic compound formed by calcium ions and nitrate ions?
A) Ca2NO3
B) Ca2NO2
C) Ca3N2
D) CaNO3
E) Ca(NO3)2
Question 44
The chemical formula for iron(II) nitrate is
A) Fe2(NO3)3
B) Fe2N3
C) Fe(NO2)2
D) Fe(NO3)2
E) Ir(NO2)2
Question 45
The Stock system name for Co2(SO3)3 is:
A) cobalt(III) sulfate
B) cobalt sulfate
C) cobalt(II) sulfate
D) cobalt(II) sulfite
E) cobalt(III) sulfite
Question 46 Which one of the following is an ion?
none of the above He
NaCl B3+
14C
Question 47 An iron(II) ion has:
A) 26 electrons and a charge of 2+
B) 24 electrons and a charge of 2+
C) 28 electrons and a charge of 2+
D) 24 electrons and a charge of 2-
E) 28 electrons and a charge of 2-
Question 48 An iron(III) ion has:
A) 23 electrons and a charge of 3+
B) 26 electrons and a charge of 3+
C) 28 electrons and a charge of 3-
D) 28 electrons and a charge of 3+
E) 24 electrons and a charge of 2-
Question 49
The Lewis structure for CS2 is:
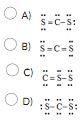
Question 50
A sulfide ion, S2-, has:
A) 16 protons and 18 electrons
B) 18 protons and 19 electrons
C) 16 protons and 8 electrons
D) 8 protons and 7 electrons
E) 10 protons and 7 electrons
Question 51
Which is the correct formula for copper(II) phosphite?
A) Cu3(PO4)2
B) Cu2PO3
C) Cu(PO3)2
D) Cu2PO4
E) Cu3(PO3)2
Question 52 Which of the following pairs of elements would be most likely to form an ionic compound?
A) Cu and K
B) Al and Rb
C) O and Zn
D) C and O
E) P and Br
Question 53
What is the correct formula for acetic acid?
CH3COOH
CH3CO2H2
CH3CO2-
CH3COOH2
Question 54
What is the correct formula for iodine monochloride?
Question 55
The correct name for Ba(OH)2 is
A) boron hydroxide
B) beryllium hydroxide
C) barium hydrate
D) barium hydroxide
E) barium hydrogen oxide
Question 56 Which one of the following is most likely to be a covalent compound?
A) Al2O3
B) CaCl2
C) CaSO4
D) KF
E) SF4
Question 57 What is the formula for the ionic compound formed by calcium and sulfur?
A) CaS2
B) Ca2S
C) CaS
D) CaS3
E) Ca3S
Question 58 Which one of the following elements is most likely to form a 2+ ion?
A) sodium
B) carbon
C) beryllium
D) fluorine
E) oxygen
Question 59 The formula for magnesium sulfate is
MgS.
MgSO4.
MnSO4.
MnS.
MnSO3.
Question 60
The electron dot formula for O2 shows
A) a double covalent bond.
B) a total of 32 electron dots.
C) an ionic bond.
D) a single covalent bond.
E) a total of 8 x 2 = 16 electron dots.