Reference no: EM131125179
1. Aluminum oxide is an ionic compound found in soils. What ions are formed by aluminum atoms and by oxygen atoms? What is the chemical formula for aluminum oxide?
2. The Fukushima nuclear disaster was more a consequence of inundation by water because of a tidal wave that followed an earthquake. This caused a large hydrogen explosion, largely due to the reaction of zirconium casings that contained the uranium fuel pellets. Using what you know about electron transfer reactions, write chemical half-reactions and the overall reaction for this disastrous event that explains the formation of hydrogen.
3.
(a) The height of an Evian bottle is approximately 25 cm. If 31.2 x 109 empty water bottles were laid end-to-end around the equator, how many times would the water bottle trail wrap around the globe. The circumference of the earth at the equator is 24,860 miles (1 mile = 1.609 km).
(b) How many times would the water bottle trail wrap around the globe if the Evian bottles were laid side-by-side?
4. For each of the half-reactions below, add the appropriate number of electrons (e) to balance the equation. Identify whether the half-reaction is an oxidation or a reduction.
5. Batteries made from mercury (Hg) are used extensively in medicine and in the electronic industries. The overall reaction can be represented by the following equation:
HgO (s) + Zn (s) → ZnO (s) + Hg (1)
(a) Using the periodic table, what is the charge on the oxide ion? What is the charge on the mercury and zinc ions in the oxide compounds? Write the chemical symbol and charge for each ion.
(b) Write the oxidation half-reaction to describe the oxidation step in the reaction. Include the necessary electrons (e-) to balance the equation.
(c) Write the reduction half-reaction to describe the reduction step in the reaction. Include the necessary electrons (e-) to balance the equation.
6. The diagram below shows an electrochemical cell made from silver (Ag) and zinc (Zn). Note the flow of electrons in the circuit.
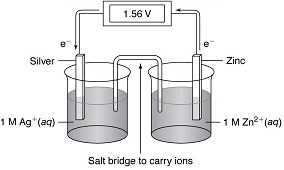
(a) What chemical species is being oxidized? Write the half-reaction for the oxidation.
(b) What chemical species is being reduced? Write the half-reaction for the reduction.
(c) Using the table of standard reduction potentials provided for Lab Project 9 or your lecture notes, calculate the voltage generated by this electrochemical cell. [Hint: All voltages listed are for the reduction half-reaction, so the equation and voltage must be reversed for the oxidation half-reaction].
(d) Combine the half-reactions to write the (balanced) chemical reaction for this electrochemical cell.
7. A History lesson
(a) Fuel cells were invented in 1839, but they were not developed into practical devices for producing electrical energy until the U.S. space program used them in the 1960's. What advantages did fuel cells have over previous sources?
(b) What was the name of the program (and its capsule) for the first U.S. manned space flight?
(c) What was the name of the program that placed U.S. astronauts on the moon?
(d) What was the name of the satellite that prompted the U.S. to embark on a serious space program? What country launched this satellite, and when?
8. Using hydrogen as a fuel has both advantages and disadvantages. Make a list of advantages and disadvantages for using hydrogen as the fuel for transportation and for producing electricity:
Transportation Advantages: Disadvantages:
Electricity Production
Advantages: Disadvantages:
9. The incomplete half reactions below in a lead-acid storage battery do not show the electrons lost or gained. The actual reactions are more complicated, but it is still possible to analyze the reactions that take place based on the half-reactions below.
Pb(s) + SO42-(aq) 4 PbSO4(s)
PbO2(s) + 4H+(aq) + SO42-(aq) 4 PbSO4(s) + 2 H20(1)
(a) Balance both equations with respect to charge by adding electrons as needed
(b) Which half-reaction represents oxidation and which represents reduction?
(c) One of the electrodes is made of lead; the other is lead dioxide. Which is the anode and which is the cathode?