Reference no: EM131355915
Question 1. (a) Give the IUPAC names of the following compounds:
(i) 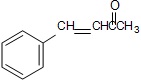
(ii) CH2 = CHCH2CHO
(iii) 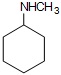
(b) Write the structures of the following compounds:
(i) 3, 4-Dibromo-2-pentanone
(ii) Butyl benzoate
Question 2. (a) Write the geometrical isomers of 2-butene and also designate them as E/Z isomers.
(b) What are various elements of symmetry? Give one example illustrating the presence (3) of such elements in them.
Question 3. Using a suitable diagram, explain the relative energies of various conformations of (5) cyclohexane.
Question 4. Explain the following terms:
(i) Auxochrome
(ii) Fingerprint region
(iii) Chemical shift
(iv) Chemical exchange
(v) Molecular ion
Question 5. What is tautomerism? Explain briefly any two types of tautomerism using suitable (5) examples.
Question 6. a) How would you prepare alkanes from the following (Give only one example)?
(i) Alkyl halide
(ii) Carboxylic acid
(iii) Alkene
b) Explain the following:
(i) In the mass spectra, alkanes give a series of peaks separated by 14 mass units.
(ii) Alkanes with odd number of carbon atoms have lower melting point than those with an even number of carbon atoms.
Question 7. a) Explain the following briefly:
(i) Alkenes are more soluble in water than corresponding alkanes.
(ii) Addition reactions of alkenes are exothermic processes.
(iii) Hydroboration appear as anti-Markownikoff's addition.
b) An alkene having molecular formula C6H12 an ozonolysis yielded butanal and (2) ethanal. What is the structural formula of alkene?
Question 8. a) Explain the following:
(i) Alkynes are more acidic than alkanes.
(ii) In NMR, the value of alkynyl proton is less than the value of alkenyl proton.
b) How would you prepare the following?
(i) 3-Octyne from 1-hexyne
(ii) 1,2-Dibromoethene from ethyne
(iii) Ethanal from ethyne
Question 9. a) Explain the following:
(i) The theoretical value of heat evolved, when hydrogens are added to benzene, is quite high as compared to the experimental value.
(ii) Nitrobenzene not undergo Friedel-Crafts alkylation.
b) Write all the possible resonance structures of cation formed from ortho nitration (1) of methyl benzene.
c) What do you understand by para-directing activators, para- directing deactivators (2) and meta-directing deactivators?
Question 10. a) Explain the following:
(i) 1-Position in naphthalene is more reactive than the 2-position towards electrophilic substitution.
(ii) Pyrrole is more basic than pyridine.
b) Predict the products of the following reactions:
(i) Oxidation of propylbenzene
(ii) Friedel-Crafts acylation of pyrrole
(iii) Friedel-Crafts alkylation of pyridine
Question 11. Complete the following reactions. Predict whether each reaction proceeds (5) predominantly by SN1 or SN2 or E1 or E2.
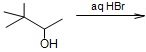
Question 12. Complete the following reaction and write its mechanism.
Question 13. Taking suitable examples discuss the Zeisel procedure used for estimation of (5) methoxy group in alkyl aryl ethers.
Question 14. Write the chemical reactions for the different methods used for the reduction of (5) aldehydes and ketones.
Question 15. Explain Fischer esterification with the help of a suitable example.
Question 16. Give the products of the following reactions:
(i) CH2 = CHCOOH →HBr ?
(ii) HOOCCH2COOH Δ→423K ?
(iii) 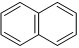 V2O5 →? hydrolysis→ ?
V2O5 →? hydrolysis→ ?
(iv) CH2CH2CN → H+/H2O
(v) CH3CHClCH2COOH → KOH?
Question 17. Explain the following reactions using suitable examples:
(i) Rosenmund reduction
(ii) Transesterification
Question 18. Give the products of reduction of nitrobenzene under acidic, alkaline and neutral (5) conditions.
Question 19. Explain the following reactions and their utility:
(i) Sandmeyer reaction
(ii) Diazo coupling
Question 20. Illustrate Edman degradation and explain its importance.
|
Create an analysis of the ethical theories the company
: You have just been hired by a company to evaluate the way the company treats its employees, suppliers, and customers. Create an analysis of the ethical theories the company would want to use to show the employees, suppliers, and customers that it ..
|
|
What are common parts shared by all different kinds of bikes
: What are the common parts shared by all the different kinds of bikes? How can you use the parts to describe the differences between the operation of one bike and another?
|
|
Are you in favor of universal databases
: The use of the Internet and web-based services has the potential to integrate not only law enforcement data, but also data from other sources and to increase the exchange of information for all law enforcement. Through the sharing of information i..
|
|
What are the major phases of an audit
: Briefly describe why on most audit engagements an auditor tests only a sample of transactions that occurred.
|
|
What is tautomerism
: What is tautomerism? Explain briefly any two types of tautomerism using suitable (5) examples - Write the chemical reactions for the different methods used for the reduction of (5) aldehydes and ketones - Explain Fischer esterification with the help ..
|
|
Determine if restrictions on elastic constants are satisfied
: The following data has been obtained experimentally for a composite based on an unidirectional carbon-epoxy prepreg. Determine if the restrictions on elastic constants are satisfied.
|
|
Define auditing attest and assurance services
: The Committee on Basic Auditing Concepts has provided a widely cited definition of auditing. What does the phrase "systematic process" mean in this definition?
|
|
Describe the effectiveness of the chosen technologies
: Criminals, law enforcement, and the public have access to many types of technology. Cell phones, G.P.S., home computers, and the Internet are commonplace. Advanced weaponry and security systems are used throughout our society as well. Technology h..
|
|
How coca-cola life based on sales decision on buyer behavior
: What stands out to you about how Coca-Cola Life based on their sales decisions on buyer behavior? Based on your reading of the eText, how do the three different types of purchasing decisions.
|