Reference no: EM132422671
Questions -
Q1. What is the dominant species (H2A, HA-, A2-) of a diprotic acid with pKa1 = 3.5 and pKa2 = 6.5 at a pH of 4.8?
Q2. Draw a Jablonski diagram. Describe the difference between the processes leading to fluorescence and phosphorescence.
Q3. Why is Raman spectroscopy using a visible light-range laser, whereas the energy of the excited vibrations lies in the infrared energy region?
Q4. X-ray absorption spectroscopy measures the absorption of X-rays by core electrons. What happens to excited core electrons? Where will you find the absorption edge of Mn 1s electrons, compared to that of Ni 1s electrons?
Q5. Describe the general composition of a chromatography instrument. Give examples for each component for an instrument that allows analyte separation by vapor pressure.
Q6. What is the separation principle in size-exclusion chromatography? In which order are analytes being eluted?
Q7. What is the purpose of isoelectric focusing in electrophoresis?
Q8. You measure a cell potential of -45mV for a WE of Ag/Ag+ in a silver ion solution of unknown concentration against a RE of Ag/Ag+ with a silver ion concentration of 0.5M. Determine the unknown Ag+ concentration.
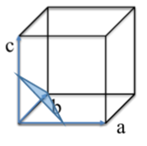
Q9. Identify the Miller indices for the plane drawn into the cubic structure below:
Q10. Given a unit cell parameter a = 4.0Å and an X-ray energy of 3 keV, calculate the diffraction angle θ for the (1 0 0) plane.
Q11. What kind of light source is used in dynamic light scattering? Which phenomenon leads to a time-dependence in the measurement?
Q12. Describe the difference between back scattered electrons and secondary electrons. Focus on the mechanism of signal formation and the difference in information that these two signals provide.
Q13. You need to determine the relative amount of proline in a mixture of amino acids. Which technique do you choose and why?