Reference no: EM131025428
Azo dyes are organic dyes that are used for many applications, such as the coloring of fabrics. Many azo dyes are derivatives of the organic substance azobenzene, C12H10N2. A closely related substance is hydrazobenzene, C12H12N2. The Lewis structures of these two substances are
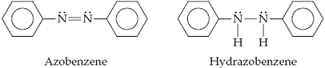
(Recall the shorthand notation used for benzene.)
(a)What is the hybridization at the N atom in each of the substances?
(b) How many unhybridized atomic orbitals are there on the N and the C atoms in each of the substances?
(c) Predict the N-C-N angles in each of the substances.
(d) Azobenzene is said to have greater delocalization of its π electrons than hydrazobenzene. Discuss this statement in light of your answers to (a) and (b).
(e) All the atoms of azobenzene lie in one plane, whereas those of hydrazobenzene do not. Is this observation consistent with the statement in part (d)?
(f) Azobenzene is an intense red-orange color, whereas hydrazobenzene is nearly colorless. Which molecule would be a better one to use in a solar energy conversion device? (See the "Chemistry Put to Work" box for more information about solar cells.)