Reference no: EM13486842
Youngsborough Products, a supplier to the automotive industry, had seen its operating margins shrink below 20% as its customers put continued pressure on pricing. Youngsborough produced four products in its plant and decided to eliminate products that no longer contributed positive gross margins. The total plant overhead cost is $122,000 per year. Details on the four products are provided here:
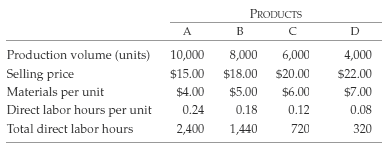
�Youngsborough calculates a plantwide overhead rate by dividing total direct labor hours into total overhead costs. Assume that plant overhead is a fixed cost during the year, but that direct labor is a variable cost. The direct labor rate is $30 per hour.
Required
(a) Calculate the plantwide cost driver rate and use this rate to assign overhead costs to products. Calculate the gross margin for each product and calculate the total gross margin.
(b) If any product is unprofitable in part a, drop this product from the mix. Recalculate the cost driver rate based on the new total direct labor hours remaining in the plant and use this rate to assign overhead costs to the remaining three products. Calculate the gross margin for each product and calculate the total gross margin.
(c) Drop any product that is unprofitable with the revised cost assignment. Repeat the process, eliminating any unprofitable products at each stage.
(d) What is happening at Youngsborough and why? How could this situation beavoided?
|
Compute the distance between first and second dark fringes
: Light of wavelength 730 nm illuminates a pair of slits separated by 0.200 mm. determine the distance between the first and second dark fringes
|
|
Explain what maximum mass of carbon dioxide
: A liquid fuel mixture contains 28.25% hexane, 14.05% heptane, and the rest octane. What maximum mass of carbon dioxide is produced by the complete combustion of 12.0kg of this fuel mixture
|
|
Obtain the wavelength of the laser light
: A laser beam is incident on two slits with a separation of 0.10 mm, and a screen is placed 4.00 m from the slits. what is the wavelength of the laser light
|
|
How about a reactor business strategy
: What are the advantages and disadvantages to an individual who accepts a job as a human resource manager in a firm that is in the midst of a retrenchment corporate strategy? How about a reactor business strategy?
|
|
What is happening at youngsborough and why
: What is happening at Youngsborough and why? How could this situation beavoided and Drop any product that is unprofitable with the revised cost assignment. Repeat the process, eliminating any unprofitable products at each stage.
|
|
Explain the concentrations of all species present
: Any feedback/how to would be great Calculate the concentrations of all species present in a 0.25 M solution of ethylammonium chloride (C2H5NH3Cl). [C2H5NH2]_____ M [H+]____M [C2H5NH3+] ___ M [Cl -] ___M [OH -] ____M
|
|
What is the water pressure at the bottom of the barrel
: A certain cylindrical drum is 3 meters in height. It filled with with water. Atmospheric pressure is 10^5 Pa, and the density of water is 1000 gl/m^3. What is the water pressure at the bottom of the barrel
|
|
Explain the ph of a 0.36 m solution of nahso4
: Calculate the pH of a 0.36 M solution of NaHSO4. If solid Na2CO3 is added to a solution of NaHSO4, what reaction occurs between the CO32- and HSO4. ions
|
|
The existence of carbonic acid molecules in aqueous solution
: When acid is added to a solution of sodium hydrogen carbonate (NaHCO3), vigorous bubbling occurs. How is this reaction related to the existence of carbonic acid (H2CO3) molecules in aqueous solution
|