Reference no: EM131637750
Assignment
Answer all questions (with full workings). Q1, Q2 each carry 5 marks; Q3 - 4 marks; Q4 - 3 marks; and Q5 - 2 marks.
1. The crystal structure of CaF2 can be simply visualized as an FCC lattice with all the tetrahedral sites occupied. If 2 Al ions and 3 Cl ions are diffused into this lattice, they sit on their corresponding cationic and anionic lattices (by knocking of equivalent number of cations and anions). Radii (in nm) of Ca, F, Al, and Cl are 0.099, 0.133, 0.051, and 0.181, respectively. Considering this:
(i) Sketch the original and the new structures (identify the position of the ions).
(ii) What is the new chemical formula of this system?
(iii) Calculate the distortion (in terms of oversize or undersize), if any, due to the presence of Cl ions instead of F ions in the new structure.
(iv) Is there any influence of the presence of Al on the answer that you got in (iii). If so, calculate the difference (with and without Al ions).
2. Draw the Fe (FCC) and Fe (BCC) unit cells to show the arrangement of atoms and the interstitial sites (both octahedral and tetrahedral sites). Based on that:
(i) Calculate how much oversize/undersize the C atom is compared to the space available if it occupies one of the sites belonging to the family of 1/2 0 0 and 1/4 1/4 1/4 in FCC, and 1/2 0 0 , 1/2 1/2 0 and 1/2 1/4 0 in BCC. Take the lattice parameter of Fe (FCC) as 0.3571 nm, Fe (BCC) as 0.2856 nm and atomic radius of carbon as 0.071 nm.
(ii) Similarly, what will be the answer if instead of C, if it is a self-interstitial defect (Fe).
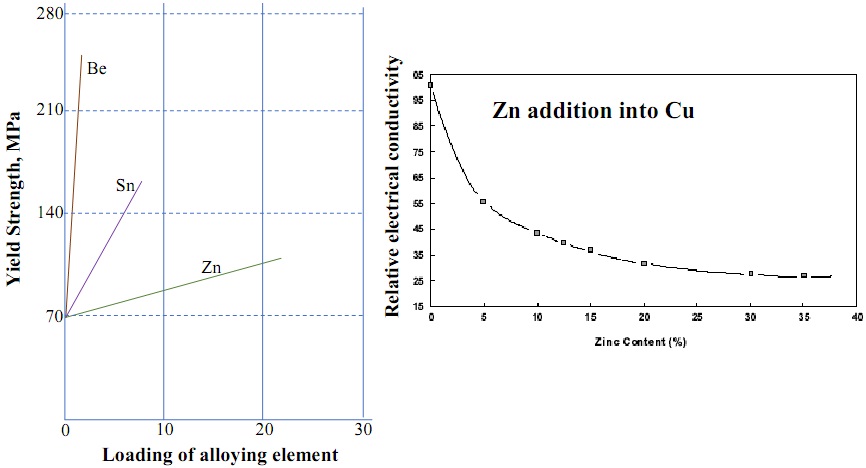
3. We have thoroughly discussed about solid solution strengthening. Considering those concepts in a Cu system, explain (i) the plot below (left-hand side) by highlighting the differences in the degree of strengthening; and (ii) the plot on the right-hand side. Take the atomic radii (in nm) of Cu, Be, Sn, and Zn as 0.1278, 0.1143, 0.1509, and 0.1332, respectively.
4. A cubic lattice is shown below. Use it to answer the following:
(i) What is the formula of the compound?
(ii) What are the fractions of octahedral and tetrahedral holes occupied?
(iii) Is this a stoichiometric compound?
5. We would like to introduce cationic vacancies and anionic vacancies in an ionic crystal like MgO. Which would you introduce as substitutional impurities: cations with a higher positive charge or lower positive charge than the cations in the parent structure; or anions with a higher negative charge or lower negative charge than the host anion? Explain your choice.