Reference no: EM13833355
Analytical Chemistry Lab
Lab 1. Volumetric Glassware Calibration
I. Introduction: This lab is to demonstrate to you the proper techniques for using analytical balance, and volumetric apparatus.
II. Analytical Balance
a. You must be familiar with the use of the analytical balance.
b. Take a piece of coin (penny, nickel, dime, or quarter). Do not handle the coin with your fingers; always use the tweezers or Kim wipe. Never directly put your coin on the analytical balance. Make sure to follow the "weighing by difference technique".
c. First zero your analytical balance carefully, and accurately weigh the weighing paper to the 0.0001 g.
d. Zero the analytical balance, put your coin on the weighing paper, and weigh them again to the 0.0001 g.
e. Calculate the mass of coin.
f. Repeat Steps "c" through "e" two more times.
g. Determine the average mass of coin and the standard deviation.
III. 25 mL Volumetric Pipet
a. Bring 250 mL beaker to obtain distilled water in the plastic container in the lab. This will be enough amounts of water for part III and IV.
b. Insert a thermometer into your container of distilled water, and measure the temperature to the 0.01 °C.
c. Calculate the density of water using a table provided. To obtain the accurate density value, simply ignore the significant figures rules at this point and copy entire digits of the answer from the calculator.
d. Obtain a "tare weight" of your 150 mL beaker to the nearest 0.0001 g using the analytical balance. The outside of container must be dry, but not for the inside.
e. Draw a few milliliters of distilled water into the pipet, rinse all internal surfaces of the pipet, and discard the rinse solution to the drain. Do not fill the pipet completely; this is wasteful, time-consuming, and inefficient. Just draw in a small amount, tilt the pipet horizontally, and turn it to rinse the sides.
f. Pipet 25.00 mL of water into the weighed receiver (150 mL beaker). Remember-Always use a pipet bulb. Never use your mouth to draw liquids into a pipet. Also do not blow out the last drop!
g. Reweigh the receiver and water.
h. Pipet a second 25.00 mL portion of water into the weighed receiver. (It is not necessary to empty the container and reweigh for the second portion; just use the second weight of the previous check as the "tare" value.)
i. Repeat on a third 25.00 mL portion.
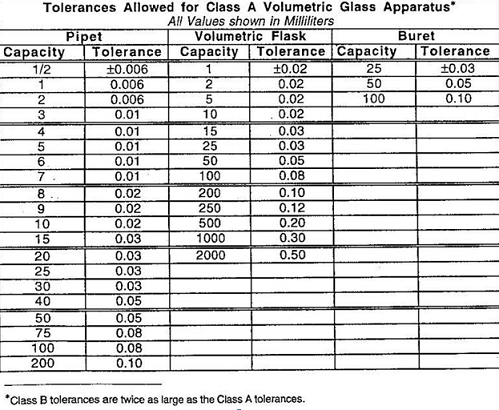
j. Determine the tolerance.
IV. 50 mL Buret
a. Obtain clean and dry 100 mL volumetric flask from your drawer, and weigh to the closest 0.0001 g using an analytical balance.
b. Mount a buret in a buret stand, and fill the buret with distilled water. Do NOT bring your buret to the balance to deliver the water into the receiver directly! Be sure that buret tip is filled and that there is no air bubbles trapped within the stopcock. Make sure the buret is absolutely vertical. If you notice that your buret is leaking, replace the buret.
c. Using the stopcock, adjust the level to the O mark (= 0.00 mL) or just below; accurately read the setting to the 0.01 mL, and record its value (initial reading).
d. Bring the weighed receiver (100 mL volumetric flask) under the tip, incline the receiver at a slight angle and touch the buret tip to the wall of the receiver. Open the stopcock and allow the meniscus to descend to a few mm above the 10 mL mark. Then very slowly set the meniscus at the 10.00 mL line. If not exactly on the line, read the setting value and record this value to the nearest 0.01 mL (final reading). Carefully touch off the tip on the wall of the receiver; move the receiver away from the tip and cover the receiver.
e. Weigh the receiver and water.
**Wait! Do not dump the water! Keep the water inside the receiver, and continue to add next 10 mL portion of water, and so on.
f. Repeat Steps "d" through "e" above for next additional 10 mL interval. Continue in 10 mL intervals in total three times. Thus the volume of water delivered from buret will be 10.00, 20.00, and 30.00 mL. Make sure to record the accurate volume of the water which you are delivering to the beaker.
V. Calculations
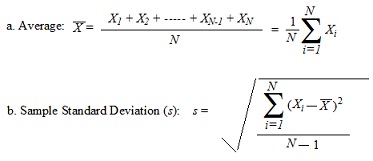
c. Calculation of Density (Use the table in the lab manual.):
(ex.) Calculate the density of water at 22.4 C.
|
Temp. (C)
|
Density (g/mL)
|
|
22.0
|
0.997799
|
|
22.4
|
?
|
|
23.0
|
0.997567
|
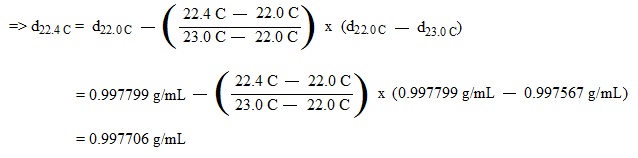
Important Laboratory Procedure
1. Weighting on the analytical Balance
Weighting by difference and quantitative transfers:
A sample is weighed by difference when a weighting or sample bottle containing the sample is weighted a small quantity of sample is transferred to a sample container (such as a volumetric flask or beaker) the bottle is reweighted and the amount of sample used is determined by the DIFFERENCE of the two weighing bottle weights.
Weighing by difference is useful when a procedure, such as titration, needs a small, known amount of sample in a container, such as a beaker, which cannot be weighted directly. Usually, the reason for not weight directly is that the container is too heavy or too big to fit on the balance. Another reason might be that the container is wet. One particular advantage to weighting by difference is that for multiple samples, the multiple containers need not each be weighted. Other reasons for not weighting directly exist, but the usual criterion for deciding to weigh by difference is whether the container which will hold the weighted sample cannot itself be weighted. A weighting bottle is to be used in the weighting by difference procedure. When weighting by difference, do not use the cover of the weighing bottle.
Some assumptions are made when weighing by difference. The first is that the sample is transferred quantitatively from the sample bottle to the sample container if some is spilled or blown away by a draft, then the amount of sample in the sample container is not the amount given by the difference in the weighing bottle weighted transferring a sample from one container to another without loss is known as quantitative transfer. A second assumption made is that the sample bottle is kept free of fingerprints. WHILE WEIGHING BY DIFFERENCE, HANDLE THE BOTTLE WITH TONGS OR A PIECE OF PAPER. Fingerprints have enough weight to ruin the results of many analytical procedures. One last assumption is that the balance zero does not change between the two weightings. The balance should not be bumped and the student should not leave the balance between the two weightings.
Finally, the number of significant figures given for a weight can save the student time, For example, if a procedure calls for 0.5gm sample, and then 0.5 ± 0.1gms are acceptable, don't waste time weighting out 0.5000gm. However, if a procedure calls for 0.500gm, then 0.500 ± 0.001 gms must be weighed out.
2. Drying Procedures
a. Open oven doors only when permitted by schedule on the door.
b. Do not dry glassware in ovens, and do not use ovens for evaporation purposes.
c. To dry a solid sample, pour the material into a glass weighing bottle and place the weighing bottle, with its lid off, in the smallest possible beaker (150 ml). put a piece of foil in the beaker with your name, section, and desk number on it. Then cover the beaker with a supported watch glass and put the assembly into the drying oven. The beaker must be covered to avoid crud disposition in the sample.
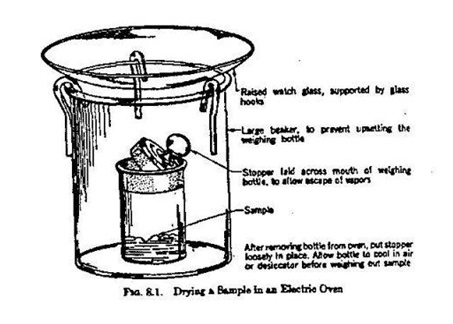
3. Desiccator
a. The desiccator does not serve to dry the sample, but merely to maintain the dryness of the sample after it has been dried. After placing a dried, hot sample into a desiccator, be sure to vent it periodically by removing the lid for a few second. If this is not done, your desiccator lid may "pop off" spontaneously due to pressure build-up.
b. Have the TA check the quantity of the desiccant, CaCl2, now in your desiccator.
c. Once the sample has been dried and placed in an efficient desiccator, it need not be dried again.
d. Do not over grease the desiccators cover so that it slides off easily. The cover is removed by sliding, not pulling.
e. It is helpful to attach the cover to the body of the desiccators with a string.
Table 1:
|
TempoC
|
Density H2O (g/ml)
|
|
18
|
0.998623
|
|
19
|
0.998433
|
|
20
|
0.998232
|
|
21
|
0.998021
|
|
22
|
0.997799
|
|
23
|
0.997567
|
|
24
|
0.997325
|
|
25
|
0.997074
|
|
26
|
0.996813
|
|
27
|
0.996542
|
|
28
|
0.996262
|
|
29
|
0.995974
|
|
30
|
0.995676
|
Table 2:
Complete a lab report and need to know the dependent and independent variables of this lab, as well as the controls and what the hypothesis would be.
- Dependent variables: calculated volume of water
- Independent variables: temperature of water/glassware
- Controls: volume of water delivered
- Hypothesis: The temperature of the room and the water will skew the results