Reference no: EM13865902
Problem 1: Hydrogen gas at a pressure of 1.25 bar is contained in a thick-walled neoprene rubber sphere which has inner and outer radii of 70 mm and 80 mm, respectively. The concentration of hydrogen at the outer surface of the sphere is negligible and leakage is so small that steady-state conditions can be assumed to occur for a long time. The solubility of hydrogen in rubber is 2.37 × 10-3 kmol m-3 (bar)-1 and the diffusivity is 1.8 × 10-10 m2 s-1. Calculate the rate at which hydrogen escapes from the sphere. (Assume the inner surface of rubber is saturated with hydrogen.)
Problem 2: (a) Ammonia gas is being absorbed by water in a wetted-wall column.
At one level of the column, the following data applies:
gas-phase mass transfer coefficient
5.22 × 10-9 kmol m-2 s-1 Pa-1
liquid-phase mass transfer coefficient 3.88 × 10-5 m s-1
Henry's constant 0.955 kPa (kmol m-3)-1.
Estimate the overall mass transfer coefficient KL.
(b) What is the ratio of the individual mass transfer resistances in Question 2 (a)?
(c) Use the following additional information to find the mass transfer flux in the column:
mole fraction of ammonia in liquid* 1.351 × 10-3
mole fraction of ammonia in gas* 0.065
total pressure of system 1.013 bar
mole mass of ammonia 17
*Note: these values will need to be converted to concentrations in kmol m-3.
(d) What is the partial pressure of the ammonia gas at the gas/liquid interface?
(e) What is the molar concentration of the ammonia in the liquid at the interface?
You will need two sheets of graph paper to complete
Problem 3: (a) Assuming that Raoult's law applies to all possible mixtures of two components, 'A' and 'B', plot a "pressure/composition" diagram at constant temperature T for the full range of mixtures from the data below. The diagram should show how the partial pressures of each component and the total pressure varies with composition.
Data:
Pure vapour pressure of 'A' at temperature T = 67 kPa
Pure vapour pressure of 'B' at temperature T = 20 kPa
(b) Using your diagram in (a), state the partial pressures and the total pressure at the reference temperature, of a mixture of 'A' and 'B' having 0.4 mole fraction 'A'.
(c) Confirm these values using Raoult's law equation.
Problem 4: A mixture of octane and nonane containing 0.70 mole fraction (m.f.) octane, at its boiling point, is to be continuously separated in a distillation column. The top product is to have a composition of 0.98 m.f. octane and the bottom product must be no richer than 0.05 m.f. octane.
The reflux ratio is set at 3 and the equilibrium data is given below.
xoct 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
yoct 0.0 0.18 0.33 0.46 0.57 0.67 0.75 0.82 0.89 0.95 1.0
(a) Determine the number of theoretical plates required to perform the operation.
(b) State which plate the feed should be introduced onto.
(c) (i) What effect would altering the reflux ratio have on the number of plates required?
(ii) In an actual industrial column, the number of plates is fixed at the design stage. In this case, what effect would a change in the reflux ratio have on the column's operation and how can this effect be used as a means of control?
(d) If a packed column were to be used, determine the height of packing required, assuming an H.E.T.P. of 1.2 m.
Problem 5: (a) Select distillation techniques for the separation of the following mixtures, giving reasons for the techniques chosen.
(i) 50 kg of a mixture of A (boiling point 160oC) and B (boiling point 405oC) where B is sensitive to heat.
(ii) 500 kgh-1 of a mixture of C (boiling point 75oC) and D (boiling point 85oC).
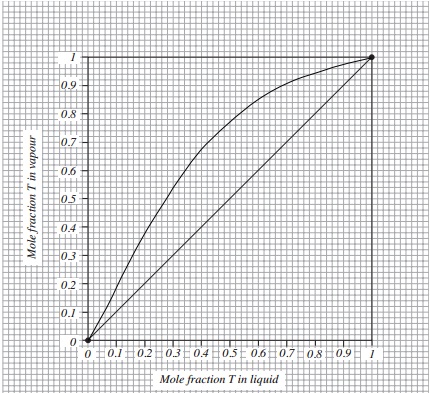
Problem 6: 100 kmol of the mixture, whose equilibrium diagram is shown in FIGURE 1, is to be batch fractionally distilled until the contents of the still have been reduced to 0.20 mole fraction T. The still initially contains 0.70 mole fraction T. The column to be used has 3 theoretical stages and will be operated with a fixed reflux ratio of 3. Determine:
(a) the amount of bottom product produced
(b) the amount and composition of the overall top product removed.
Hint: you will need to choose final distillate values between 0.6 and 0.99 mole fraction T.