Reference no: EM131102036
1.The bonding in diamond is
A) Ionic
B) Covalent
C)Metallic
D) Van der Waals
2.Given that the electronegativity of Mg is 1.2 and the electronegativity of O is 3.5, the fraction of bonding of MgO that is ionic
A) 100%
B) 54%
C) 73%
D) 0%
3. The number of protons in an atom is equal to
A) the atomic number
B) the atomic mass
C) the number of electrons
D)the number of neutrons
4. An element has a valence of 2 and an atomic number of 27. Based only on the quantum numbers, how many electrons must be present in the 3d energy level?
A) 7
B) 5
C) 2
D) 1
5. Would you expect Al2O3 or aluminum (Al) to have the higher modulus of elasticity?
A) Aluminum
B) Al2O3
C) They have equal modulus of elasticity
6. For the cubic crystal structure, which structure has the largest Atomic Packing factor (APF)?
A) FCC
B) SC
C) BCC
7. For BCC metal with lattice parameter a0 = 0.3294 nm in cm is and one atom per lattice point, the atomic radius
A) 1.73´10-8 cm
B) 1.16 ´10-8 cm
C) 1.43´10-8 cm
D) 2 ´10-8 cm
8. Calculate the theoretical density of BCC iron (atomic weight is 55.847 g/mol) with lattice parameter a0 = 0.2866 nm
A) 7.88 g/cm3
B) 1.56 g/cm3
C) 9.45 g/cm3
D) 8.89 g/cm3
9. Naturally occurring Cu has an atomic weight of 63.55. Its isotopes are Cu63 and Cu65. What is the abundance (in atomic percent) of Cu63?
A) 50%
B) 72.5%
C) 25.5%
D) 100%
10. The Miller indices of the direction in the lattice below are
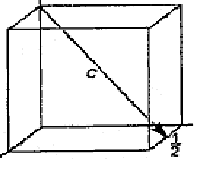
A) [001]
B) [120]
C) [1 22]
D) [122]
11. Please circle the true statements regarding the Miller indices
A) A direction and its negative are equivalent
B) A plane and its negative are equivalent
C) A direction and its multiple are equivalent
D) A plane and its multiple are equivalent
12. A sample of cubic SiC was analyzed using X-Ray Diffraction (XRD). It was found that the (111) peak was located at 2θ of 16°. The wavelength (λ) of the x-ray radiation used in this experiment was 0.6975 Å. The lattice constant (ao) of this form of SiC is
A) 11.00 Å
B) 5.50 Å
C) 4.34 Å
D) 8.68 Å
13. Given the atomic weight of copper ACu= 63.5 g/mol, the density ?Cu= 8.4 g/cm3, and the activation energy Qv= 20000 cal/mol, the equilibrium number of vacancies in Cu at 1000oC is
A) 20000
B) 6.5 ?1023 vacancies/cm3
C) 5.8 ?1019 vacancies/cm3
D) 2.9 ?1019 vacancies/cm3
14. If we introduce one carbon atom for every 100 iron atoms in an interstitial position in BCC iron, giving a lattice parameter of 0.2867 nm, the density of the Fe-C alloy is
A) 3.90 g/cm3
B) 15.50 g/cm3
C) 7.89 g/cm3
D) 31.00 g/cm3
15. Which of the following statements are true for the line defect
A) Burger vector is parallel to dislocation in an edge dislocation
B) Burger vector is perpendicular to dislocation in an edge dislocation
C) Burger vector is perpendicular to dislocation in a screw dislocation
D) Burger vector is parallel to dislocation in a screw dislocation
16. Consider the (111) slip plane and [0 1 1] slip direction for a single crystal of copper. A tensile stress (σ) of 3000 psi is applied to this crystal along the [001] direction. What is the resolved shear stress (τr) along the slip direction?
A) τr = 4,565 psi
B) τr = 3,000 psi
C) τr = 39,412 psi
D) τr = 1225 psi
17. For the effect of crystal defect on material strength, which statement is true?
A) Defect does not affect the material strength
B) Defect decrease the material strength
C) Defect increase the material strength
18. Compare the activation energy for the diffusion of H in FCC iron and that for self-diffusion in FCC iron
A) The activation energy for the diffusion of H in FCC iron is equal to that for self-diffusion in FCC iron
B) The activation energy for the diffusion of H in FCC iron is larger than that for self-diffusion in FCC iron
C) The activation energy for the diffusion of H in FCC iron is smaller than that for self-diffusion in FCC iron
19. BCC iron foil with thickness of 0.001-cm is used to separate a high concentration hydrogen gas from a low concentration hydrogen gas at 650°C. 5×108 hydrogen (H) atoms/cm3 are in equilibrium on one side of the foil, and 2×103 hydrogen (H) atoms/cm3 are in equilibrium with the other side. The flux of
hydrogen through the foil is
A) 2.55 ×108 H atoms/(cm2•s)
B) 2.55 ×108 H g/(cm2•s)
C) 0.84 ×108 H atoms/(cm2•s)
D) 0.84 ×108 H g/(cm2•s)
20. BCC iron containing 0.05% C is heated to 912°C in an atmosphere that produces 1.20% C at the surface and is held for 24 hours. Calculate the carbon content at 0.05 cm beneath the surface
A) 1.11% C
B) 5.56% C
C) 0.01% C
D) 0.02% C
21. If 10 hours are required to successfully carburize a batch of 500 steel gears at 900°C, where the iron has the FCC structure, how long does it take if the carburizing temperature is increased to 1000°C?
A) 3.3 hours
B) 5.2 hours
C) 9 hours
D) 10 hours
22. A polymer bar's dimensions are 1 in x 2 in x 15 in. The polymer has a modulus of elasticity of 600,000 psi. What force is required to stretch the bar elastically to 15.25 in.?
A) 10,000 lb
B) 20,000 lb
C) 30,000 lb
D) 40,000 lb
23. A 3-in. diameter rod of copper is to be reduced to a 2-in. diameter rod by being pushed through an 6 opening. The modulus of elasticity for the copper is 17 x 10 psi and the yield strength is 40,000 psi. To account for the elastic strain, the diameter of the opening would be
A) 2.521 in
B) 3.0 in
C) 0.895 in
D) 1.995 in
24. If an 850-lb force is applied to a 0.15-in diameter nickel wire having a yield strength of 45,000 psi and a tensile strength of 55,000 psi, what will occur?
A) The wire will plastically deform
B) The wire will experience necking
C) The wire will break
D) The wire will elastically deform
25. Based on Hume-Rothery's rules, gold (Au) and silver (Ag) would be expected to display
A) limited solid solubility
B) unlimited solid solubility
C) no solubility
D) partial solubility
26. Diffusion mechanism typically includes
A) Vacancy diffusion
B) Substitutional diffusion
C) Interstitial diffusion
D) Atomic jump
27. Based on the Cu-Ni phase diagram below, the phase compositions for a Cu-40% Ni alloy at 1250°C are
A) L: 40 wt% Ni and ?: 40 wt% Ni
B) L: 45 wt% Ni and ?: 45 wt% Ni
C) L: 45 wt% Ni and ?: 32 wt% Ni
D) L: 32 wt% Ni and ?: 45 wt% Ni
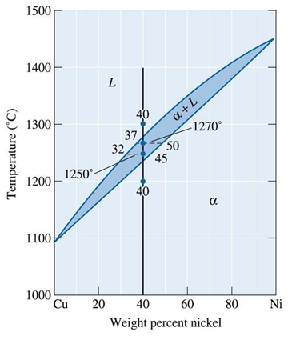
The equilibrium phase diagram for the Cu-Ni system
28. Based on the Cu-Ni phase diagram above, how many grams of nickel must be added to 500 grams of copper to produce an alloy with liquidus temperature of 1350°C?
A) 750 grams of nickel
B) 200 grams of nickel
C) 1000 grams of nickel
D) 542 grams of nickel
29. Based on the Cu-Ni phase diagram in the last page, what are the liquidus temperature and solidus temperature of 40 wt% nickel for the Cu-Ni alloy?
A) Liquidus temperature = 1230°C and solidus temperature = 1280°C
B) Liquidus temperature = 1230°C and solidus temperature = 1300°C
C) Liquidus temperature = 1200°C and solidus temperature = 1300°C
D) Liquidus temperature = 1280°C and solidus temperature = 1230°C
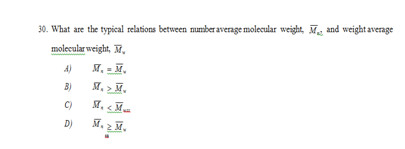
31. In the condensation polymerization generating 6,6-Nylon, it creates
A) no byproduct
B) byproduct of water (H2O)
C) byproduct of CH3
D) byproduct of CO2
32. The monomer of polyethylene is
A) CH3
B) CH2CH2
C) CH4
D) CH2
33. A polyethylene sample contains 8000 chains with molecular weights between 5000 and 10,000 g/mol, and 16000 chains with molecular weights between 10,000 and 20,000 g/mol. The number average molecular weight of the polyethylene is
A) 15,000 g/mol
B) 7,500 g/mol
C) 10,000 g/mol
D) 12,500 g/molAppendices
1. Periodic Table
2. Selected Physical Properties of Some Elements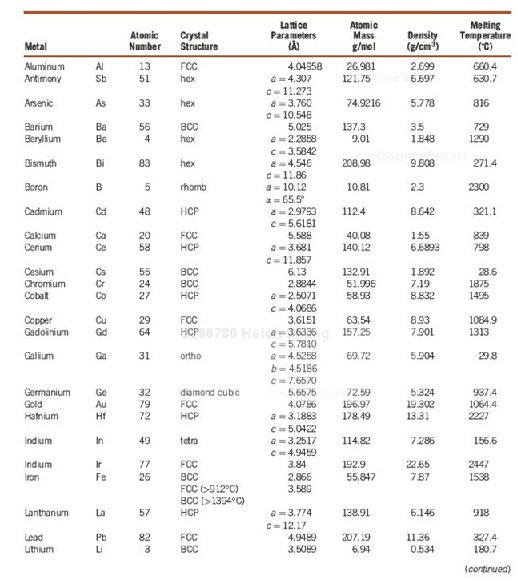

3. The Atomic and Ionic Radii of Selected Elements
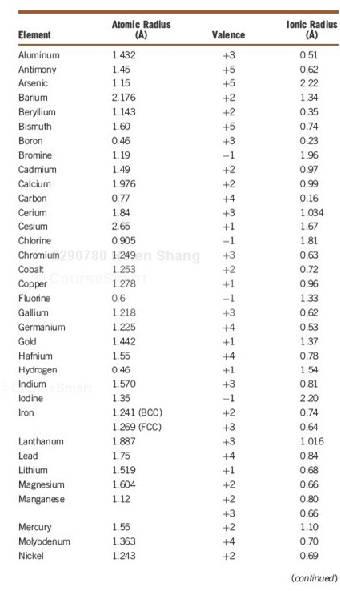
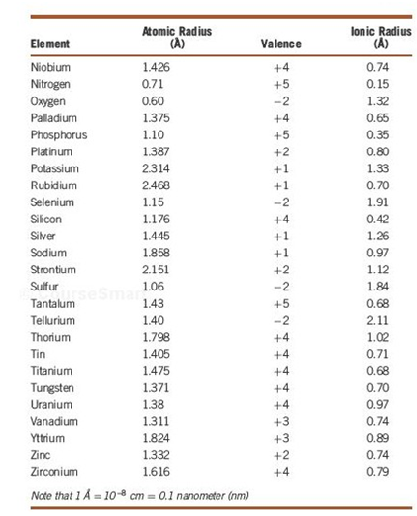
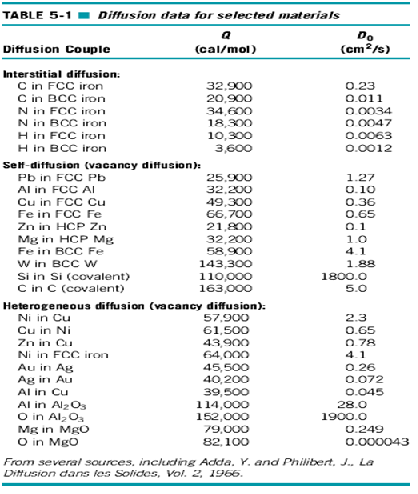
4. Diffusion data for Selected Materials
5. The Value of Error function
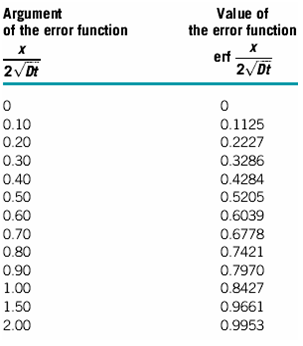
6. Avogadro's number NA=6.023 ×1023
7. Ideal gas constant R= 1.987 cal / (mol•K)
8. 1 psi = pounds per square inch