Reference no: EM131093691
Questions 1 - 3: MATCH a structure or term from the following list with each description below. Place the letter of the structure or term in the blank to the left of the description.
a. benzyne e. +NO
b. +NO2 f. Meisenheimer complex
c. R3C+ g. 
d. electron-donating h. electron-withdrawing
1. The reactive electrophile in Friedel-Crafts acylation reactions.
2. The electrophile in aromatic nitration.
3. Intermediate in the elimination-addition mechanism of nucleophilic aromatic substitution.
Questions 4 - 6: Consider the Friedel-Crafts alkylation reaction below to answer the following question(s):
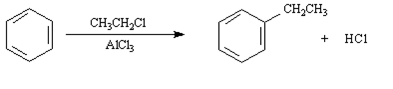
1. Draw the structure of the electrophilic intermediate in this reaction.
2. What is the role of the AlCl3 in the reaction?
3. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structures.
Questions 7 and 8: Consider the data below to answer the following question(s).
The -NH2 group is listed in our textbook as the strongest o,p-directing activator in electrophilic aromatic substitution reactions. However, when aniline is subjected to standard nitration conditions poor yields of m- nitroaniline result.
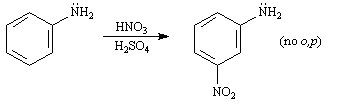
1. Clearly, the reaction conditions are influencing the directing effect of the -NH2 group. Explain why this occurs, using both words and structures.
Questions 9 and 10: Consider the reaction below to answer the following question(s).
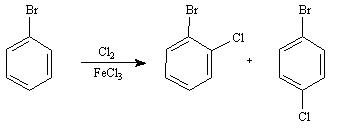
1. Write the complete stepwise mechanism for the formation of the ortho product. Show all intermediate structures and show all electron flow with arrows.
2. Draw resonance structures for the intermediate carbocation that explain the directing effect of the -Br.
Questions 11: Rank the compounds in each group below according to their reactivity toward electrophilic aromatic substitution (most reactive = 1; least reactive = 3). Place the number corresponding to the compounds' relative reactivity in the blank below the compound.
Attachment:- 1_Organic Chem_Sample.pdf