Reference no: EM13934638
A dodecane C12H26 used in jet engine is burned with 0% excess air. The products of combustion is then expanded through a variable area duct. write a simple program (use excel sheet or any other software) to Draw the area ratio versus Mach number for isentropic flow (0.1<Mac3.0). Take the increment in Mach number as 0.1. Compare the trend of the graph with that of air as a perfect gas
Solution Procedures:
1. Write down the stoichiometric and actual combustion equations of the fuel/air mixture.
2. Determine the percentage of each component of product.
3. Use table (A-21) in your thermodynamic text book to evaluate the average specific heat (Cp) for each component.
4. Estimate Cp and R for the mixture.
5. Then evaluate the specific heat ratio (k).
6. Employ the calculated specific heat ratio in Eq. 1.17 to determine the area ratio.
7. Tabulate the area ratio vs. Mach number (use the range and increment stated in the lecture slides). Preferable Excel Sheet
8. Repeat 6 and 7 for Eq. 1.18.
9. Draw one graph illustrating the Area ratio as a function of Mach number for Eq.s 1.17 and 1.18 tables.
10. Summarize your impression on the trend of curves and the influence of k.
Recommendations:
1. After completing step 5, I suggest that you show me your evaluated k because if it's wrong you will loose.
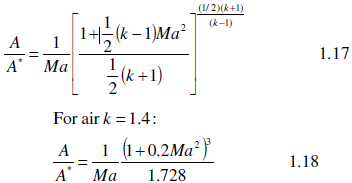
TABLE A: Variation of c-p with Temperature for Selected Ideal Gases
c-p/R = α + βT + γT2 + δT3 + εT4
T is in K, equations vaild from 300 to 1000 K
| Gas |
α |
β x 103 |
γ x 106 |
δ x 109 |
ε x 1012 |
| CO |
3.71 |
-1.619 |
3.692 |
-2.032 |
0.24 |
| CO2 |
2.401 |
8.735 |
-6.607 |
2.002 |
0 |
| H2 |
3.057 |
2.677 |
-5.81 |
5.521 |
-1.812 |
| H2O |
4.07 |
-1.108 |
4.152 |
-2.964 |
0.807 |
| O2 |
3.626 |
-1.878 |
7.055 |
-6.764 |
2.156 |
| N2 |
3.675 |
-1.208 |
2.324 |
-0.632 |
-0.226 |
| Air |
3.653 |
-1.337 |
3.294 |
-1.913 |
0.2763 |
| SO2 |
3.267 |
5.324 |
0.684 |
-5.281 |
2559 |
| CH4 |
3.826 |
-3.979 |
24.558 |
-22.733 |
6.963 |
| C2H2 |
1.41 |
19.057 |
-24.5 |
16.391 |
-4.135 |
| C2H4 |
1.426 |
11.383 |
7.989 |
-16.254 |
6.749 |
| Monatomic gases° |
2.5 |
0 |
0 |
0 |
0 |