Reference no: EM13843791
1. State the hybridization of the nitrogen atoms in urea, shown below:
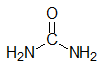
A. sp3 hybridized
B. sp2 hybridized
C. sp hybridized
D. not hybridized
E. this concept does not apply here
2. Which of the following best describes the compound shown below:
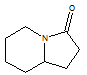
A. ketone B. tertiary amine C. 2º amine D. ester E. 3º amide
3. What best describes the term"degeneracy"?
A. Two orbitals have the same shape
B. Two orbitals merge into one
C. An orbital is so small it can't hold any electrons
D. Two orbitals have identical energy levels
E. An orbital has spherical shape
4. Consider pyridine-N-oxide. This as an overall uncharged molecule. Which of the proposed structures are compliant with basic rules of valence and bonding? (If you work this problem on the computer, you may have to zoom to >180% to see the lone pairs properly)
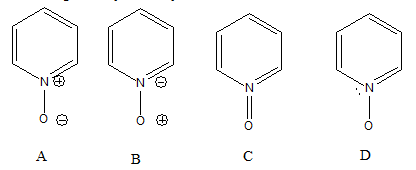
A. Only structure A
B. Only structure B
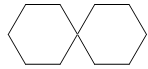
C. Only structure C
D. Only structure D
E. C and D are both compliant.
5. Use your model kit to determine how many of the molecules below are physically possible (can actually exist)
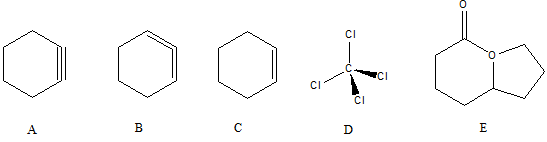
A. A-C can exist, but not the others
B. Only E can actually exist
C. D and E can exist, but not the others
D. Only C can exist
E. C and D can exist, but not the others
6. Which of the following contain(s) polar covalent bonds?
A. NH3
B. Na2O
C. H2
D. KF
E. both A and C
7. What is the proper molecular formula for the compound below?
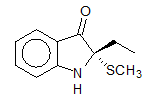
A. C11H15NOS B. C11H13NOS C. C10H10NOSCH3 D. C10H13N1O1S1 E. none of these
8. Which statement is TRUE?
A. In its ground state, beryllium can form a maximum of two bonds
B. A carbon atom hybridizes because a hybridized carbon atom is lower in energy than in its ground state
C. In contrast to carbon, oxygen cannothybridize.
D. Electrons have much less mass than protons and neutrons
E.Hydrocarbons, which contain sp hybridized carbon will also contain a triple bond
9. Consider the molecule below and select the best description of this molecule (build a model!)
A. Its central carbon must be sp2 hybridized to accommodate its shape
B. The molecule is twisted propeller-like around the central carbon
C. This molecule has a lone pair, not shown here
D. The central carbon of this molecule violates the octet rule
E. The molecular formula of this molecule is C9H8.
10. Consider the atomic orbital energy diagram below and select the most appropriate statement:
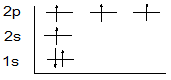
A. This represents the ground state of nitrogen
B. This represents sp3 hybridized carbon
C. This represents anexcited, but unhybridized, state of carbon
D. This represents sp hybridized carbon
E. The diagram, as shown, violates the Pauli exclusion principle
11. Which molecule contains an sp-hybridized carbon?
A. HCN
B. CH2=CH2
C. CH3Cl
D. acetone (find its fomula on the internet!)
E. CH3CH3
12. Consider the molecules below and select one option:
A. All of them have permanent dipole moments
B. 1, 2, and 4 have permanent dipole moments
C. Only 4 has a permanent dipole moment
D. 1 and 2have permanent dipole moments
E. None of them have permanent dipole moments
13. In which of the following does the central atom have 2 pairs of non-bonding electrons?
A. HCN
B. CO2
C. CO32-
D. NH4+
E. CH3OCH3
14. Which of the following violate the octet rule?
1. borane (BH3), 2. carbon monoxide 3. ozone (O3), 4. atmospheric oxygen(O2, also called dioxygen)
A. All of them
B. Ozone and borane
C. Carbon monoxide and O2
D. Borane and O2
E. None of them
15. The C-N-Obond angle in the following molecule would be expected to be approximately:
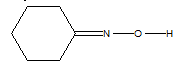
A. 90º
B. 109 º
C. 120 º
D. 145 º
E. 180 º
16. What is the relationship of the molecules below?
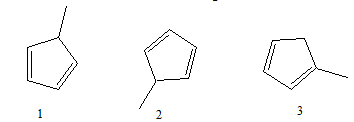
A. They all are constitutional isomers
B. 1 and 2 are resonance forms, 2 and 3 are constitutional isomers
C. They all are resonance forms
D. 1 and 2 are constitutional isomers while 3 is not an isomer of 1 and 2
E. 1 and 2 are identical while 3 is a constitutional isomer of 1 and 2
17. Which is NOT a correct Lewis structure?
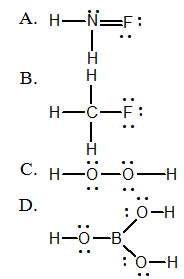
E. They are all validLewis structures
18. Of the following solvents which one has a non-zero dipole moment?
A. benzene
B. Cyclohexane
C. Diethyl ether
D. Cyclopentane
E. None of these have dipole moments
19. Give the general formula for a cyclic alkene.
A. CnH2n-4
B. CnH2n-2
C. CnH2n
D. CnH2n+2
E. CnH2n+1
20. Which functional group is NOT contained in prostaglandin E1?
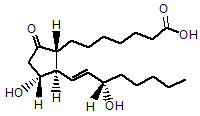
A. Ketone
B. 2 º alcohol
C. 3 º alcohol
D. Carboxylic acid
E. Alkene