Reference no: EM13793118
Spectrophotometric Determination of Iron in Dietary Tablets
Objectives:
(1) The principle behind UV-visible spectrophotometry. Components of an UV-visible spectrophotometer.
(2) Beer's law. Construction of calibration curve.
Instrumentation:
A Cary 100 Bio UV-Visible spectrophotometer will be used for the unknown sample and the iron(II) standards.
Principle
In this experiment, Fe3+ in an unknown sample is converted to Fe2+ which reacts with 1,10-phenanthroline to form the tris(1,10-phenanthroline) iron (III) complex (see the second reaction shown in Scheme 1). The absorbance of the complex is then measured with UV-Visible spectrophotometry and the concentration of the complex is deduced from the calibration curve constructed from measuring the standard solutions. Hydroquinone is used as the reducing agent to reduce Fe3+ to Fe2+. Other reducing agents such as hydroxylamine can also be used.
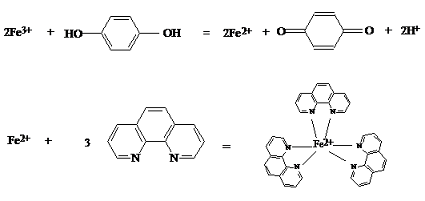
Scheme 1. Complexation of Iron (II) with 1,10-phenathroline and Reduction of Iron (III) with Hydroquinone
Suggested Procedure:
Solutions:
(1) Prepare a standard iron solution by weighing 0.0702 g ferrous ammonium sulfate and transferring the weighed sample to a 1-L volumetric flask. Add deionized water to dissolve the solid. This is then followed by adding 2.5 mL concentrated sulfuric acid and deionized water to the mark. The resulting solution contains 10.0 mg iron.
(2) Dissolve 100 mg 1,10-phenanthroline in a 100-mL volumetric flask. Transfer the solution to a plastic bottle for storage.
(3) Prepare a 25-mL fresh hydroquinone solution containing 0.25 g hydroquinone. Store this solution in dark. Also weigh 0.625 g sodium citrate and dissolve it in a 25-mL volumetric flask.
Solution Preparation:
(1) Into a series of 100-mL volumetric flasks, add with pipets 1.00, 2.00, 5.00, 10.00, and 25.00 mL of the standard Fe2+ solution. Put 50 mL distilled water in another 100-mL volumetric flask for a blank. In each flask, add sodium citrate dropwise with a disposable pipet until the solution pH is about 3.5 (it would take about 30 drops).
(2) Now add 2 mL hydroquinone and 3 mL 1,10 phenanthroline into each flask. Dilute to mark and mix these solutions thoroughly.
(3) Unknown digestion. Place one tablet in a 100-mL beaker and boil gently in the fume hood with 6 M HCl for 15 min. Filter the solution (some of the binding material in the tablet will not dissolve) into a 100-mL volumetric flask. Wash the beaker and filter several times with small portions of distilled water to complete a quantitative transfer. Let the solution cool, dilute to the mark, and mix well.
(4) Due to the high concentration of unknown sample, a further dilution is required before measurement. Dilute your unknown solution which obtained from the previous step by factor of 100. Transfer 1 mL of the digested unknown solution into a 100mL volumetric flask and add sodium citrate until the solution pH is around 3.5. Again add 2 mL hydroquinone and 3 mL 1,10-phenanthroline.
(5) Allow all of the above solutions to stand for 15 min. In the meantime, become familiar with the use of the Perkin-Elmer 6 UV-Vis spectrophotometer (see Appendix A2b or the instruction taped on the top panel of the instrument).
(6) Choose the standard solution that has the intermediate concentration and measure the spectrum of the tris(1,10-phenanthroline) iron (III) complex. Once you identify the peak and the wavelength, set the instrument at the wavelength and measure the absorbances of all the standards and the unknown.
(7) Use Microsoft Excel (Appendix 1) to make a graph of absorbance versus ppm of Fe in the standards. Based on the resultant calibration curve, deduce the concentration of Fe in the original tablet.
UV-Visible Spectrophotometer Operational Instruction
1. Turn on the computer.
2. Turn on the main power of "CARY 100 Bio UV-Visible Spectrophotometer" (on the bottom left of the instrument).
Part 1: Determine the Maximal Absorption Wavelength of Your Solution
(1) Launch the Scan Program on the desktop by double-clicking the "Scan" icon.
(2) Click "Setup" icon located at the top left corner of the window.
(3) After the Setup window pops up, change the parameters of the measurement.
(3a) Select "Cary" tab, choose the wavelength range from 700 nm to 300 nm.
(3b) Select "Options" tab, switch the "Beam mode" from "double" to "single
front."
(3c) Select "Baseline" tab, choose "Zero/baseline correction."
(3d) Please DO NOT change other parameters in this window.
(4) Wait about 10 seconds for the instrument to set up the parameters, then click "Zero" icon in order to zero the background of the instrument.
(5) After clicking the "Baseline" icon, you will see "insert a blank sample into the sample compartment and press the OK button to collect the 100%T baseline scan." Please insert your blank, then click "OK."
(6) After the measurement of your blank sample, another dialog will pop up which states, "Block the sample beam and press OK to collect the 0%T baseline scan." Put in the black metal plate, then click "OK."
(7) After the "Start light" turns green, take out of the black metal plate. After this, you are ready to measure your sample (use 0.5 ppm Fe solution).
(8) Click "Start," a "Save As" window will pop up, please save your file under: desktopàfolder " Chem462 Spring 201X"àfolder "Group#."
(9) A report will automatically show at the bottom of the window.
(10) After you are done with the measurement, please close the window.
Part 2: Determine the Absorption of Your Unknown Sample
(1) Launch the Concentration Program on the desktop by double-clicking the "Concentration" icon.
(2) Click the "Setup" icon located on the top left corner of the window.
(3) After the Setup window pops up, change the parameters of the measureme
(3a) Select the "Cary" tab, choose the wavelength corresponding to the
maximalabsorption wavelength, which you obtained during the "scan" proceduredescribed above). Set up "Sample/Std Averaging" to 3. The system will read your sample and standards three times.
(3b) Select the "Standards" tab, set up the concentration unit, order of your
standards and concentration of your standards. Fit Type should be set up to
"Linear".
(3c) Select the "Sample" tab, choose the numbers of your sample.
(3d) Select the "Options" tab, switch the "Beam mode" from "Double Beams"
to "Single Beam"
(4) After the "Start light" turns green, you are ready to measure your sample.
(5) Click "Start", a "Standard/Sample Selection" window will pop up, click "OK".
(6) Next, a "Save As" window will pop up, please save your file under: desktopàfolder " Chem462 Spring 201X"àfolder "Group#"à file name
(7) After the "Present Standard" window pops up, load your sample in the sample cell, and then click "OK". Repeating the same step for all your standards and unknown solution.
(8) When the experiment is finished, close the program and turn off the instrument.
(9) PLEASE WRITE DOWN ALL YOUR DATA IN YOUR NOTE BOOK, JUST IN CASE SOMEONE ACCIDENTALLY DELETES YOUR FILE.
Note: For the spectra collected under the "scan" procedure in the first part of the experiment, please save the same file as the "CSV" format, which can be directly imported into Excel for plotting the absorption spectra.
Questions:
1. What are the roles of sodium citrate and hydroquinone? What would happen without them?
2. There are many other methods that can be used to measure iron. List 3-4 of them.
3. What would happen to the calibration curve if the concentration of the tris(1,10-phenanthroline) iron (III) complex is very high?
4. Nickel can also form complexes with 1,10-phenanthroline. If you want to use this spectrophotometric method to analyze iron in a sample that also contains nickel, think of a simple method that will allow you to circumvent the problem.