Reference no: EM132957136
SEM202 Thermodynamics - Deakin University
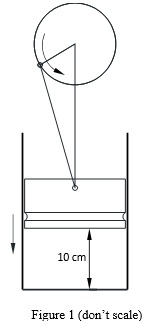
Question 1. a. The piston-cylinder device contains fresh charge (air and fuel mixture) at 313 oK and an atmospheric pressure of 100 kPa (see Figure 1). Initially, the piston height is 10 cm from the cylinder head, as shown in the figure. The piston moves downward 3 cm, compressing the charge isothermally. The piston area is X mm2. Determine the work done by the piston-cylinder device in terms of Joules.
b. Determine the work done if the charge is compressed adiabatically. Show both processes on a P-V diagram comparing both processes.
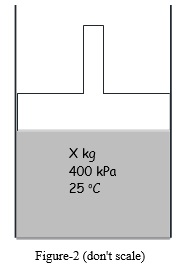
Question 2. A frictionless piston-cylinder device contains X kg of air at 400 kPa and 25 oC, as shown in Figure 2. The piston carries a static load, including its mass, of 20 kN, but is otherwise unconnected to an external system. The air is heated by a resistive heater placed inside the cylinder until the piston is twice as high as it was initially. If the atmospheric pressure is 101 kPa, how much work is done on the air? Give a schematic diagram of the process indicating two states on a P-V diagram. (Given that R=0.287 kJ/kg-K, absolute temperature 0 oK = -273 oC and g = 9.81 m/s2 )
Question 3 The pressure-vessel A has a volume X m3 and is connected to vessel B, as shown in Figure 3. Vessel A contains steam at 200 oC, 85% vapor and 15% liquid by volume, while vessel B is evacuated.
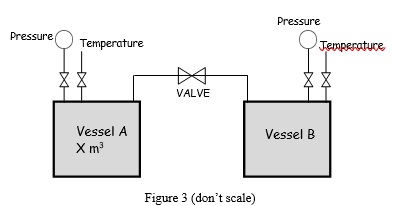
The valve is then opened, and both vessels eventually come to a steady pressure of 400 kPa
and 200 oC. Heat is transferred to maintain the constant temperature (200 oC).
Determine:
(i) The quality, internal energy and enthalpy of the vapor before and after the steady- state is reached.
(ii) The volume of vessel B.
(iii) Show the process on a T-v diagram with respect to saturation lines.
Use the last three digits of your student ID as the volume of vessel A.
(For Example: If your Student ID is 218360568, last 3 digits = 568, X=0.568 m3)
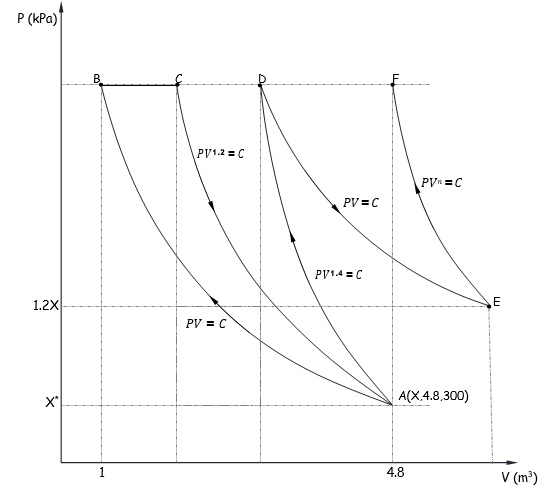
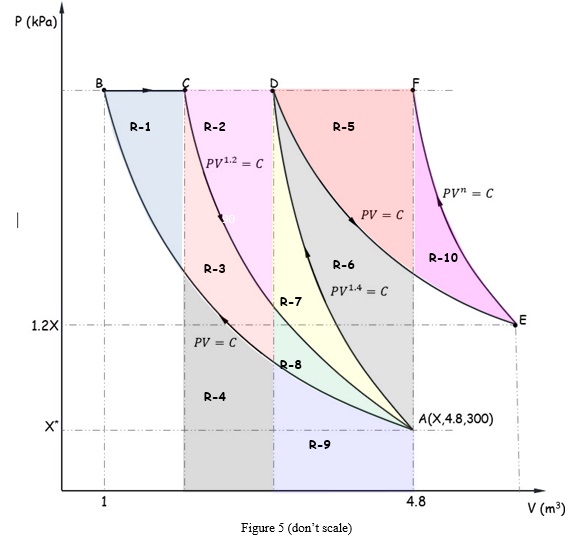
Question 4. An ideal gas (R = 0.287 kJ/kg-K) goes from state A to state F via six different processes (ABCADEF), as indicated in the P-V diagram (Figure 4). The pressure, volume and temperature at state A given as X kPa, 4.8 m3 and 300 oK respectively. The pressure at state E is 20% higher than the pressure at State A. Determine the polytropic index of the process EF and the temperature at state F (Given that the volume at F is same as the volume at state A).
Figure
b. Calculate the area (kJ) of the region marked in Figure 5.
Attachment:- Thermodynamics.rar
|
Identify the total for income tax expense
: Identify the total for income tax expense that would be reported in the Statement of Profit or Loss and Other Comprehensive Income for the period
|
|
Explain how to calculate the ratio
: Explain how to calculate the ratio, what it measures, and what it tells an analyst about the company. Finally, select a company's recent financial statements
|
|
What rate should use when evaluating the new project
: Safe Division has a new project that is similar to their usual projects. What rate should they use when evaluating the new project?
|
|
How much were the expenses for the year
: If Henry and Kate's Company has $300,000 of sales revenue, pays $50,000 in dividends and has net income of $100,000, how much were the expenses for the year?
|
|
SEM202 Thermodynamics Assignment
: SEM202 Thermodynamics Assignment Help and Solution, Deakin University - Assessment Writing Service - Determine the work done if the charge is compressed
|
|
Calculate the number of outstanding shares from information
: Calculate the number of outstanding shares from the information given. Show your calculations. The information relates to the number of common shares
|
|
What is total stockholders equity at the company fiscal year
: What is the total stockholders' equity at the company's fiscal year end? On January 1, 2020, Mega Company started the year with a $250,000 credit balance
|
|
Calculate total number of outstanding shares of grant corp
: If Grant Corporation has 120,000 shares of common stock authorized, Calculate the total number of outstanding shares of Grant Corporation.
|
|
What is the amount of capital in excess of par
: What was the amount of retained earnings at the beginning of the year? What is the amount of capital in excess of par? Compute earnings per share.
|