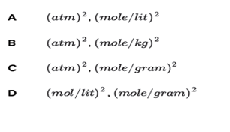Reference no: EM13869193
QUESTION 1
Seema visited a Natural Gas Compressing Unit and found that the gas can be liquefied under specific conditions of temperature and pressure. While sharing her experience with friends she got confused. Help her to identify the correct set of conditions
A Low temperature, low pressure
B High temperature, low pressure
C Low temperature, high pressure
D High temperature, high pressure
QUESTION 2
Which of the following statements are true for pure substances?
(i) Pure substances contain only one kind of particles
(ii) Pure substances may be compounds or mixtures
(iii) Pure substances have the same composition throughout
(iv) Pure substances can be exemplified by all elements other than nickel
A (i) and (ii)
B (i) and (iii)
C (iii) and (iv)
D (ii) and (iii)
QUESTION 3
A mixture of sulphur and carbon disulphide is
A heterogeneous and shows Tyndall effect
B homogeneous and shows Tyndall effect
C heterogeneous and does not show Tyndall effect
D homogeneous and does not show Tyndall effect
QUESTION 4
Which of the following is an element?
A Sand
B Diamond
C NaCl
D Glass
QUESTION 5
Which of the following statements is not true about an atom?
A Atoms are not able to exist independently
B Atoms are the basic units from which molecules and ions are formed
C Atoms are always neutral in nature
D Atoms aggregate in large numbers to form the matter that we can see, feel or touch
QUESTION 6
Find out the correct sentence about manure
(i) Manure contains large quantities of organic matter and small quantities of nutrients.
(ii) It increases the water holding capacity of sandy soil.
(iii) It helps in draining out of excess of water from clayey soil.
(iv) Its excessive use pollutes environment because it is made of animal excretory waste.
A (i) and (iii)
B (i) and (ii)
C (ii) and (iii)
D (iii) and (iv)
QUESTION 7

An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change ?
A Baking powder
B Lime
C Ammonium hydroxide solution
D Hydrochloric acid
QUESTION 8
Which of the following property is generally not shown by metals ?
A Electrical conduction
B Sonorous in nature
C Dullness
D Ductility
QUESTION 9
According to Mendeleev's periodic law, the elements were arranged in the periodic table in the order of
A increasing atomic number
B decreasing atomic number
C increasing atomic masses
D decreasing atomic masses
QUESTION 10
Organic acids are
A weak electrolyte.
B strong electrolyte.
C non electrolyte
D none of the above.
QUESTION 11
The conjugate base of HSO4 - in aqueous solution is
A SO42-
B H3SO4
C H2SO4
D None of these
QUESTION 12
When Δ n = 2, the units atm of Kp and Kc are..