Reference no: EM131008810
Question:
a. Propose a structure for the compound.
b. Assign the 1H NMR and 13C NMR spectra for each compound
On the next four pages are MS, IR, and 1H and 13C NMR spectra of compounds A, B, C and D. The molecular formulas are given. Use these spectra to propose structures forA, B, C and D. For each compound do the following:
a. Propose a structure for the compound.
b. Assign the 1H NMR and 13C NMR spectra for each compound.
Note: if you are not confident with your proposed structure, try your best to identify as many as possible functional groups (fragments) on MS, IR, 1H NMR and 13C spectra to gain credits.
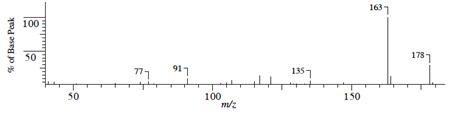
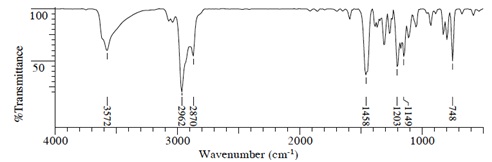
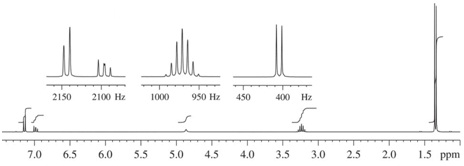
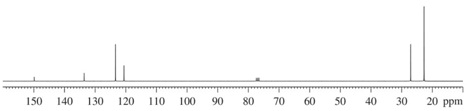
Compound A (C7H12O)
(1) Mass Spectrum
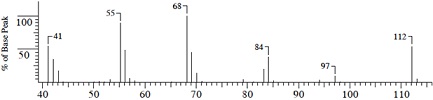
(2) IR Spectrum
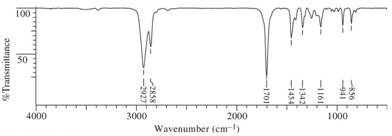
(3) 1H NMR Spectrum
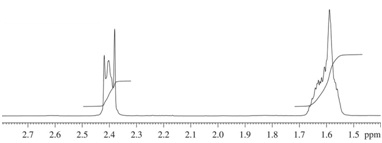
(4) 13C NMR Spectrum
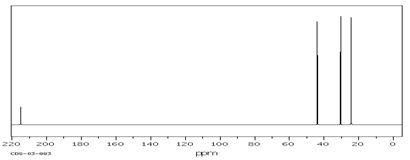
Compound B (C5H8O)
(1) Mass Spectrum
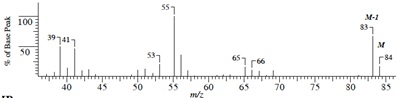
(2) IR Spectrum
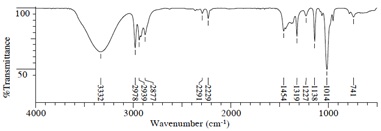
(3) 1H NMR Spectrum
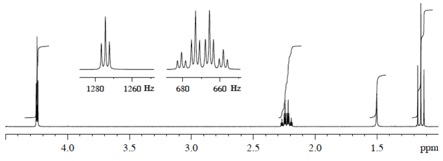
(4) 13C NMR Spectrum
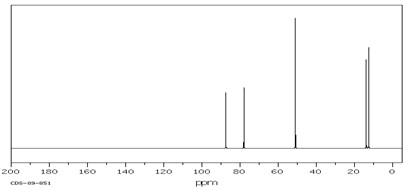
Compound C(C9H14O)
(1) Mass Spectrum
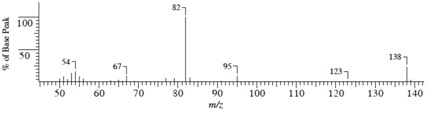
(2) IR Spectrum
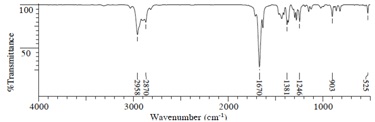
(3) 1H NMR Spectrum
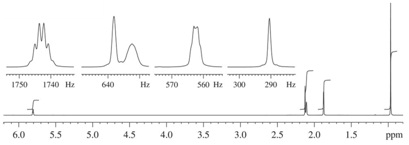
(4) 13C NMR Spectrum (in CDCl3)
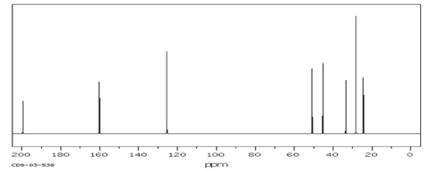
Compound D(C12H18O)
(1) Mass Spectrum
(2) IR Spectrum
(3) 1H NMR Spectrum
(4) 13C NMR Spectrum
|
Brief description of two neurological factors
: Brief description of two neurological factors that may be related to criminal behavior. Then, describe what potential they each demonstrate for predicting criminal behavior and explain why, using specific examples
|
|
Problem regarding the confidence intervals
: Several factors are involved in the creation of a confidence interval. Among them are the sample size, the level of confidence, and the margin of error. Which statements are true?
|
|
Problem regarding the sure of getting
: Recently, two students made worldwide headlines by spinning a Belgian euro 250 times and getting 140 heads-that's 56%. That makes the 90% confidence interval (51%, 61%). What does this mean? Are the conclusions in parts a-e correct? Explain your a..
|
|
How many music players will be bought
: How many music players will be bought at a price of $260? Graph the demand function for 0 ≤ x ≤ 400. Find the marginal demand, q'(x). Interpret the meaning of the derivative.
|
|
Propose a structure for the compound
: Propose a structure for the compound - assign the 1H NMR and 13C NMR spectra for each compound
|
|
Define suburban sprawl
: Define suburban sprawl and describe how human population growth causes it. List the negative, environmental impacts caused by suburban sprawl
|
|
Problem regarding the catalog sales
: A catalog sales company promises to deliver orders placed on the Internet within 3 days. Follow up calls to a few randomly selected customers show that a 95% confidence interval for the proportion of all orders that arrive on time is 88% ; 6%. Wha..
|
|
Problem regarding the mn ore conditions
: Consider each situation described below. Identify the population and the sample, explain what p and p represent, and tell whether the methods of this chapter can be used to create a confidence interval.
|
|
Problem regarding the margin of error
: A corporate executive reports the re- sults of an employee satisfaction survey, stating that 52% of employees say they are either "satisfied" or "extremely satisfied" with their jobs, and then says "the margin of error is plus or minus 4%." Explai..
|