Reference no: EM132676997
CHT353 Drug Targets - Cardiff University
Question 1. Tetracycline is a widely used antibiotic for which resistance is now widespread. This drug inhibits bacterial protein synthesis by binding to the bacterial ribosome, although experiments show that tetracycline binds to human ribosomes with similar affinity! Propose a reason to explain why the drug tend to accumulate in bacterial rather than human cells.
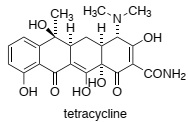
Question 2. The flavin-dependent enzyme TetX catalyses the oxidation of tetracycline to yield an inactive form of the drug (shown below) in a reaction that also requires molecular oxygen (O2) and the co-factor NADPH:
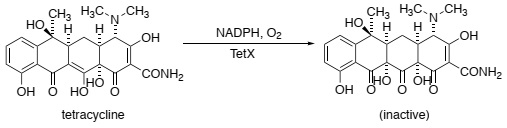
Briefly explain why oxidised tetracycline binds less tightly to the ribosome (think about metal ions).
Question 3. Write a curly-arrow mechanism to show how tetracycline reacts with the intermediate that is formed in the active site of TetX (shown below) to yield the oxidised product.
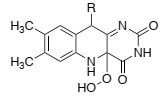
Question 4. Briefly describe one other molecular mechanism that bacteria use to become resistant to tetracycline.
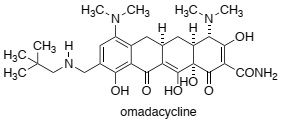
Question 5. Tetracycline exhibits an MIC for tetracycline-resistant Staphylococcus aureus of greater than 64 mg/mL. The corresponding MIC for omadacycline (shown below) is 0.1 mg/mL. Define the meaning of MIC and briefly discuss how omadacycline overcomes both resistance mechanisms discussed in questions 2 and 4.
Virus-encoded enzymes as drug targets
Question 6. The following thermodynamic cycle can be written that provides an insight into how tightly enzymes bind to transition states.
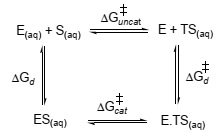
Given that kuncat = -RTexp(-ΔG‡uncat/RT) and Kd = -RTexp(-ΔGd/RT), prove that the following expression is correct:
K‡d/Kd = kuncat/kcat
where kuncat and kcat are the turnover numbers of the uncatalyzed reaction in water and of the enzyme-catalysed reaction, respectively, and Kd is the dissociation constant for the ES complex.
Question 7. Experiments on a wide variety of enzymes show that the ratio kuncat/kcat has an average value of 10-12, and Km values are approximately 10-6 M. Using these values, calculate the approximate free energy (ΔG‡d) with which the TS "binds" to the enzyme in water.
Question 8. The influenza virus encodes an enzyme called neuraminidase, which catalyses the following reaction.
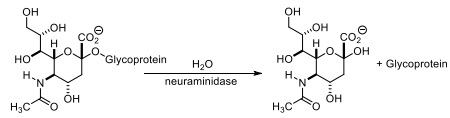
Tamiflu (shown below) is a pro-drug that is converted by an esterase into its active form within human cells. The active form of the drug inhibits neuraminidase in cells that are infected with influenza virus.
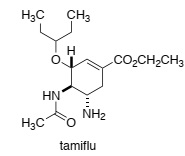
By drawing the transition stage for the first step of the reaction that is catalysed by neuraminidase, briefly explain why the active form of tamiflu binds to the enzyme with sub- nanomolar affinity.