Reference no: EM133127440
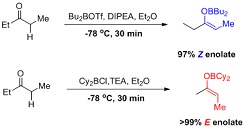
Question 1. Explain the different outcomes of the following boron mediated enolate formation?
Question 2. Glucolipsin A, a glycolipid possessing glucokinase-activating properties, was discovered at Bristol-Myers Squibb, but the absolute stereochemistry of the natural product remined elusive. Fürstner and co-workers elucidated the absolute stereochemistry via synthesis and spectroscopic analysis of the natural macrolide and its C2-symmteric stereoisomers. In their approach, the utilized the Evans aldol reaction that provided the aldol product with good yield and excellent diastereo selectivity.
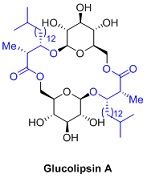
Fürstner, A. et al. J. Org. Chem. 2004, 69, 459-467.
Propose a method to synthesize the blue partin Glucolipsin A, using Evans's aldol reaction?Show reaction mechanism and explain the stereochemical outcome of the reaction.
Question 3. D. L. Boger et al. reported the total synthesis of Bleomycin A2. They devised an efficient synthesis for the construction of the tripeptide S, tetrapeptide S and pentapeptide S subunits of the natural product. In their strategy, they utilizedthe Evans aldol reactionwithN-Boc-D-alaninal that provided the aldol product with good yield and excellent diastereo selectivity.
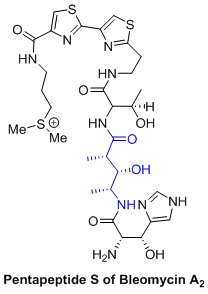
Boger, D.L. et al. J. Org. Chem. 1992, 57, 4331-4333.
Propose a method to synthesize the blue partin Bleomycin A2 A, using Evans's aldol reaction? Show reaction mechanism and explain the stereochemical outcome of the reaction.
Attachment:- Glycolipid possessing.rar
|
How would Carter record this transaction
: The Carter Corporation purchased $2,000 of direct materials on account from its largest vendor. How would Carter record this transaction
|
|
Calculate the current yield of this debenture
: The bonds have a face value of $1,000 and a yield to maturity of 9%. Calculate the current yield of this debenture
|
|
What is the total cost per unit
: Overhead was applied at a predetermined rate of $4 per dollar of direct labor costs. Job 1245 is part of a batch containing 100 units. What is the total cost
|
|
Prepare a retained earnings statement for the month of june
: On June 1, 2022, Waterway Industries was started with an initial investment in the company of $25,194 cash. Prepare a retained earnings statement for the month
|
|
Propose a method to synthesize the blue partin Bleomycin
: Propose a method to synthesize the blue partin Bleomycin A2 A, using Evans's aldol reaction? Show reaction mechanism and explain the stereochemical outcome
|
|
Prepare moonwalker journal entries for purchase
: Prepare Moonwalker's journal entries for: (a) purchase of the investment and; (b) receipt of annual interest and discount amortization
|
|
How much will monica have in her retirement account
: Monica is planning to contribute $200 at the start of the month with immediate effect to her RRSP account. How much will Monica have in her retirement account
|
|
Calculate the depletion
: The purchase price plus additional costs necessary to prepare the mine for extraction of the coal totaled $5,640,000. Calculate depletion for 2021
|
|
Compute the annual net cash inflows for the project
: The investment required to get the project underway will amount to $2,400,000 in machinery. Compute the annual net cash inflows for the project
|