Reference no: EM13278192
The following molecule is chloroethane.
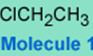
It is unreactive towards hydroxide and most nucleophiles under normal conditions, i.e., the chlorine-carbon bond is not a very good electrophilic center, hence 1 is not a good alkylating agent and does not transfer the CH3CH2 group easily.
On the other hand, there is a class of drugs that contain a chloroethyl group which are good alkylating agents and have been used clinically to treat cancer. An example of this class of drugs is melphalan.
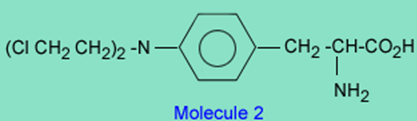
The reason melphalan, is a good alkylating agent, and 1 is not, is due to the formation of the intermediate 3 caused by the close proximity of a basic nitrogen to the carbon-chlorine bond in 2. Reaction of 3 with a nucleophile (Nuc) gives the alkylated nucleophile, 4.
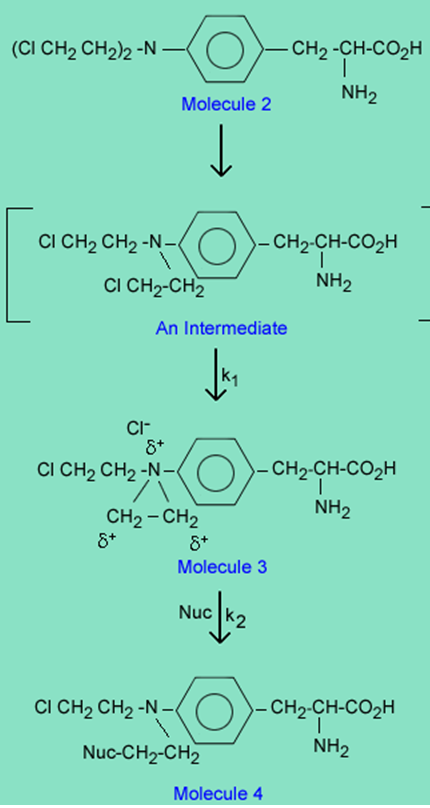
In vivo, in a therapeutic setting, the Nuc is specifically a nitrogen or oxygen on a purine in a DNA strand, which once alkylated cleaves from the DNA strand to create errors in reading the DNA code and ultimately death of a cancer cell. The alkylation takes place in two steps defined by the rate constants k1 and k2.
1. If k1 > k2, is the overall reaction of molecule 2àmolecule 4 an SN1 or SN2 reaction?
2. Conversely, if k2 > k1 is the overall reaction SN1 or SN2? Explain why.
3. If the basic pKa of the nitrogen in the -N-(CH2 CH2 Cl)2 in molecule 2 is 1.5 and the basic pKa in the -N-(CH2 CH2 Cl)2 in Molecule 5 is 6.5, which drug, (molecule 2 or molecule 5) is more likely to go by an SN1 reaction with the nucleophile and why? Note that the rate or ease of reaction k1 for 2à3 will depend on how basic is the nitrogen in -N-(CH2 CH2 Cl)2.
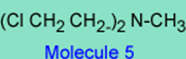
Assignment Two:
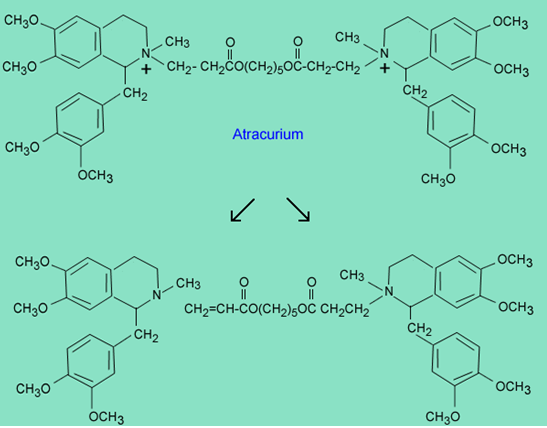
The following molecule, atracurium, is a very complex drug. It is a neuromuscular blocking agent and is related to the natural product tubocurarine. Atracurium exhibits a very short half-life (20-min). The products of the fastest inactivation process are shown below.
Explain what mechanism is responsible for the formation of the products.
Hint: The acidic pKa of CH3(C=O)CH3àCH3(C=O)CH2 - is 20.
|
Calculate its rate of radiation into the surrounding space
: A box-shaped wood stove has dimensions of 0.75 m x 1.2 m x 0.40 m an emissivity of 0.80, Calculate its rate of radiation into the surrounding space
|
|
What range of sampling frequency allows exact reconstruction
: An analog signal contains frequencies up to 10kHz. (a) What range of sampling frequencies allows exact reconstruction of this signal from its samples. (b) Suppose that we sample this signal with a sampling frequency Fs = 8kHz.
|
|
What is the maximum rise in temperature of the water
: From a height of 35.5m , a 1.50kg bird dives (from rest) into a small fish tank containing 53.5kg of water. What is the maximum rise in temperature of the water
|
|
Compute the ph of a solution made acetate
: Calculate the pH of a solution made by adding 24 g of sodium acetate, NaCH3COO, to 26 g of acetic acid, CH3COOH, and dissolving in water to make 200. mL of solution.
|
|
Products of the fastest inactivation process
: The products of the fastest inactivation process-Explain what mechanism is responsible for the formation of the products.
|
|
Find the corresponding increment in collector current
: Consider an NMOS transistor having kn=10mA/V2. Let thr transistor be biased at Vov= 0.5V. If a 0.05-V signal is superimposed on VGS, find the corresponding increment in collector current by evaluating the total collector current iD.
|
|
What is its acceleration at its peak height
: You toss a ball up in the air with an initial velocity of 8 m/s (immediately after leaving your hand). Use gravity = -9.8 m/s2. What is its acceleration at its peak height
|
|
Evaluate the equilibrium pressure of hcl
: In the reaction below, 3.84 atm each of H2 and Cl2 were placed into a 1.00 L flask and allowed to react: H2(g) + Cl2(g) 2 HCl(g) Given that Kc = 62.1, calculate the equilibrium pressure of HCl.
|
|
Design circuit that will take 2-clocks with different pulses
: Design a circuit that will take two clocks with different pulses as an input, determine within one second the faster clock and lights an led to denote the faster clock. The led should stay on until it is reset by a control signal.
|