Reference no: EM13488926
Presented below is an amortization schedule related to Spangler Company's 5-year, $100,000 bond with a 7% interest rate and a 5% yield, purchased on December 31, 2010, for $108,660.
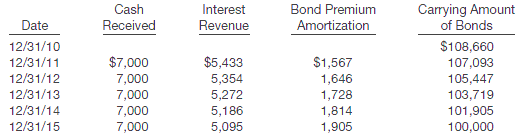
�The following schedule presents a comparison of the amortized cost and fair value of the bonds at year-end.
�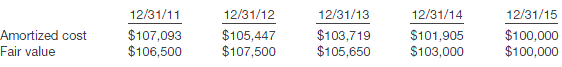
Instructions
(a) Prepare the journal entry to record the purchase of these bonds on December 31, 2010, assuming the bonds are classified as held-to-maturity securities.
(b) Prepare the journal entry(ies) related to the held-to-maturity bonds for 2011.
(c) Prepare the journal entry(ies) related to the held-to-maturity bonds for 2013.
(d) Prepare the journal entry(ies) to record the purchase of these bonds, assuming they are classified as available-for-sale.
(e) Prepare the journal entry(ies) related to the available-for-sale bonds for 2011.
(f) Prepare the journal entry(ies) related to the available-for-sale bonds for2013.
|
What would be the annual breakeven point in units sold
: If sales commissions are discontinued and fixed salaries are raised by a total of $81,000, what would be the annual breakeven point in (a) units sold and (b) revenues?
|
|
Compute the energy released
: Calculate the energy released (per mole of deuterium consumed) for the following fusion reaction, 2/1 H + 3/1 H -> 4/2 He + 1/0 n given the following molar masses of nucleons and nuclei
|
|
What will be the store operating income
: If 35,000 units are sold, what will be the store's operating income (loss)?
|
|
Compute the activity for cs2 at mole fraction
: Calculate the activity for CS2 at mole fraction= .722 for a Henry's Law standard state using the following data: 446.9 Torr and kH(CS2)=1750 Torr
|
|
Prepare the journal entry to record the purchase
: Prepare the journal entry to record the purchase of these bonds on December 31, 2010, assuming the bonds are classified as held-to-maturity securities.
|
|
What is the annual breakeven point
: What is the annual breakeven point in (a) units sold and (b) revenues?
|
|
How to calculate the ph of the solution
: determine the major species present after the reaction is complete d. calculate the pH of the solution Complete these steps for the addition of 1.0 mL , 25.0 mL, 50.0mL, 100.0 mL , 110.0 mL and 1.0 M KOH.
|
|
Define cvp analysis
: CVP analysis, shoe stores. The WalkRite Shoe Company operates a chain of shoe stores that sell 10 different styles of inexpensive men's shoes with identical unit costs and selling prices.
|
|
Evaluate the specific heat of the metal
: Calculate the specific heat of the metal and its approximate atomic mass. Suggest a name for the unknown metal. Specific Heat: atomic mass: element
|