Reference no: EM13520172
The Bear Motel opened for business on May 1, 2012. Its trial balance before adjustment on May 31 is as follows
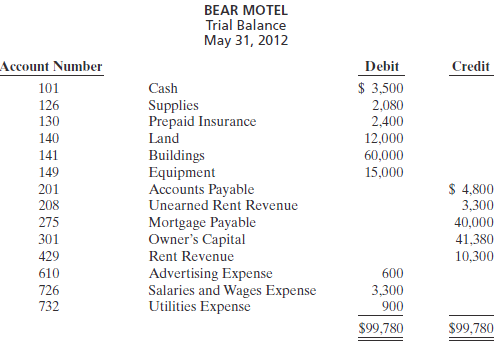
In addition to those accounts listed on the trial balance, the chart of accounts for Bear Motel also contains the following accounts and account numbers: No. 142 Accumulated Depreciation- Buildings, No. 150 Accumulated Depreciation-Equipment, No. 212 Salaries and Wages Payable, No. 230 Interest Payable, No. 619 Depreciation Expense, No. 631 Supplies Expense, No. 718 Interest Expense, and No. 722 Insurance Expense.
Other data:
1. Prepaid insurance is a 1-year policy starting May 1, 2012.
2. A count of supplies shows $750 of unused supplies on May 31.
3. Annual depreciation is $3,000 on the buildings and $1,500 on equipment.
4. The mortgage interest rate is 12%. (The mortgage was taken out on May 1.)
5. Two-thirds of the unearned rent revenue has been earned.
6. Salaries of $750 are accrued and unpaid at May 31.
Instructions
(a) Journalize the adjusting entries on May 31.
(b) Prepare a ledger using the three-column form of account. Enter the trial balance amounts and post the adjusting entries. (Use J1 as the posting reference.)
(c) Prepare an adjusted trial balance on May 31.
(d) Prepare an income statement and an owner's equity statement for the month of May and a balance sheet at May31.
|
Explain the number of photons numerically
: How many photons are required to heat 275 mL of coffee from 25.0 C to 62.0 C. Assume that the coffee has the same density, 0.997 g/mL, and specific heat capacity, 4.184 J/(g K), as water over this temperature range. Express the number of photons n..
|
|
What is the first excited state of this atom
: He+ is a helum atom with one electron missing. Treating this like a hydrogen arom, what is the first excited state of this atom
|
|
Explain rate constant for a particular second-order reaction
: The rate constant for a particular second-order reaction is 0.47 M-1 s-1. If the initial concentration of reactant is 0.25 mol/L, it takes _____ s for the concentration to decrease to 0.13 mol/L.
|
|
Erp software for qriosity inc.
: Qriosity Inc., a company of less than 100 seats, is in need of suitable ERP software. The company having had a long, fruitful relationship with Microsoft for more than 10 years, wants to buy a Microsoft ERP product? Which of the following is su..
|
|
Prepare a ledger using the three-column form of account
: Prepare a ledger using the three-column form of account. Enter the trial balance amounts and post the adjusting entries. (Use J1 as the posting reference.)
|
|
Compute rate constant and half- life for the radium decay
: Calculate the rate constant and half- life for the radium decay. Starting with 1.0 g of the radium sample, what is the activity after 500 years? The molar mass of ra-226 is 226.03 g/mol.
|
|
What environmentally friendly packaging strategies
: 1. What environmentally friendly packaging strategies might a firm adopt?
|
|
Define what is the molarity of tris
: A bioengineer preparing cells for a cloning experiment bathes a small piece of rat epithelial tissue in a TRIS buffer, (HOCH2)3CNH2. The buffer is made by dissolving 43.0 g TRIS (pKb = 5.91) in enough 0.095 M HCl to make 1.00 L of solution. What i..
|
|
Adjusted prior to the preparation of the statements
: Why do you think the loan officer suspected that the accounts had not been adjusted prior to the preparation of the statements and indicate possible accounts that might need to be adjusted before an accrete set of financial statements could be prep..
|