Reference no: EM13528473
Imperial Carpet has the following unadjusted trial balance as of March 31, 2012.
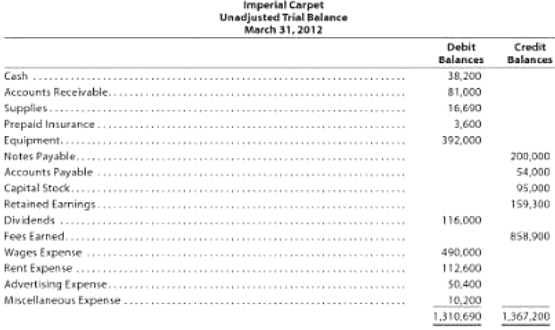
The debit and credit totals are not equal as a result of the following errors:
a. The balance of cash was understated by $12,000.
b. A cash receipt of $13,900 was posted as a debit to Cash of $19,300.
c. A debit of $15,000 to Accounts Receivable was not posted.
d. A return of $90 of defective supplies was erroneously posted as a $900 credit to Supplies.
e. The balance of Notes Payable was understated by $35,200.
g. A credit of $7,600 in Accounts Payable was overlooked when determining the balance of the account.
h. A debit of $10,000 for dividends was posted as a credit to Retained Earnings.
i. The balance of $116,200 in Rent Expense was entered as $112,600 in the trial balance.
j. Gas, Electricity, and Water Expense, with balance of $48,300 was omitted from the trial balance.
Instructions
1. Prepare a corrected unadjusted trial balance as of March 31, 2012.
2. Does the fact that the unadjusted trial balance in (1) is balanced mean that there are no errors in the accounts? Explain.
|
Explain the stepwise dissociation constants
: Calculate the pH of a 0.20 M H2SO3 solution that has the stepwise dissociation constants Ka1 = 1.5 × 10-2 and Ka2 = 6.3 × 10-8.
|
|
Determine how big was the force
: You are playing golf with a friend on a scenic course in the Rocky Mountains near Denver. When your friend misses an easy putt, How big was the force
|
|
The evolution of galapagos finches
: The evolution of Galapagos finches: a. How are Darwin’s Galapagos finches good examples of natural selection and adaptive radiation? b. How can changes in beak morphology in Galapagos finches potentially lead to new species?
|
|
Explain three moles of c(s) are heated from 300k to 600 k
: Three moles of C(s) are heated from 300K to 600 K at constant P. Over the temperature range, Cp = -12.19 + 0.1126 T/K - 1.947 x 10-4 T^2/K^2. Find delta H and delta S.
|
|
Prepare a corrected unadjusted trial balance
: Prepare a corrected unadjusted trial balance as of March 31, 2012 and does the fact that the unadjusted trial balance in (1) is balanced mean that there are no errors in the accounts? Explain.
|
|
Find out the equilibrium constant for the system n2o4
: Determine the equilibrium constant for the system N2O4 2NO2 at 25 degrees C. The concentrations are shown here: [N2O2] = 3.78 x 10^-2 M, [NO2] = 1.41 x 10^-2 M.
|
|
Find the appropriate banking angle of car
: If a roadway is banked at the proper angle, a car can round a corner without any assistance from friction between the tires and the road
|
|
What kingdom has been termedas paraphyletic and why
: what kingdom has been termedas paraphyletic and why? How is evolution demonstrated by the fossil record, anatomy, development, and biochemistry/ molecular biology?
|
|
Evaluate the delta g standard and kc for the reactions
: Under standard state conditions, what spontaneous reaction will occur in aqueous solution among the ions Ce4+, Ce3+, Ge3+ and Fe2+. Include the state of each species. Iron: and cerium: Also Calculate the Delta G standard and Kc for the reactions
|