Reference no: EM13990551
Acrolein (C3H40) is a specialty chemical intermediate used in the manufacture of acrylic acid (C3H4O2) and the synthesis of methionine, an essential amino acid. It is generated via the catalytic oxidation of propylene with air in the presence of steam at a temperature in the range 350-450t." A flowchart of the process is shown below for an assumed basis of calculation of 100 kmol propylene fed.
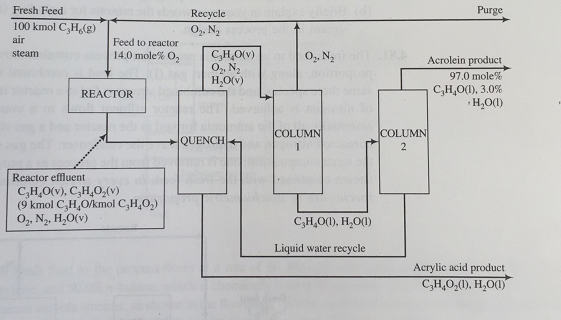
The molar ratios of propylene to oxygen to steam in the fresh feed to the reactor are 10:20:6.5. of the propylene fed, 90.0% is converted to acrolein and the rest is converted to acrylic acid.
C3H6 + O2 → C3H4O4+ H2O
C3H6+ (3/2) O2→ C3H4O2 + H2O
To meet the stoichiometric requirements of the process and remain outside of the flammable zone, the oxygen concentration entering the reactor is maintained at 14.0 mole%.
A water quench followed by a pair of separation columns is used to isolate and purify the acrolein product In the quench tower, all of the acrylic acid from the reactor effluent is absorbed in liquid water recycled from Column 2. Of the water entering the quench tower in the reactor effluent and recycle streams, half emerges with the acrylic acid liquid stream and the other half emerges in the gas stream fed to Column 1.
The oxygen and nitrogen entering Column 1 emerge in the product stream leaving the top of the column, from which a fraction Zp, is taken off as purge while the remainder is recycled to the reactor. The acrolein and water entering Column 1 are recovered in a liquid stream leaving the bottom of Column 1 and sent to Column 2. The product stream from the top of Column 2 is a liquid mixture containing 97.0 mole% acrolein, which represents essentially all of the acrolein entering the column, and 3.0 mole % water. The product stream leaving the bottom of Column 2 is essentially pure liquid water and is the recycle stream fed to the quench tower.
(a) Completely label the flowchart for the process. Describe in your own words the purpose of each process unit.
(b) Perform a degree-of-freedom analysis for the system and write the equations you would solve to calculate the values of all unknown variables on the flowchart (including the purge fraction).
(c) Complete the solution.