Reference no: EM13895118
For the following reactions:
a. Specify which of the following equations represent oxidation-reduction reactions.
b. Write the balanced half-reactions for the reactions you identified as redox in part a.
c. Identify the species being oxidized and the species being reduced in part b.
1) For the following reactions:
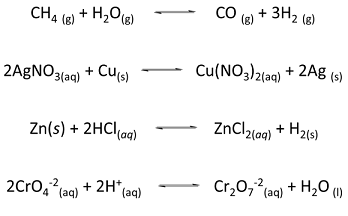
For the following reactions:
a. Specify which of the following equations represent oxidation–reduction reactions.
b. Write the balanced half-reactions for the reactions you identified as redox in part a.
c. Identify the species being oxidized and the species being reduced in part b.
2) Given the following data:
|
ΔG0 (kJ/mol)
f
|
|
H2O(l)
|
-237
|
|
H2(g)
|
0
|
|
OH-(aq)
|
-157
|
|
e-
|
0
|
a. Balance the following half-reaction.
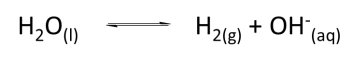
a. Estimate ε0 for the half-reaction.
2) Consider a membrane in which leakage gates allow K+ to diffuse out of the cell.
a. The inside concentration of potassium is 100mM, and the outside concentration is 10mM. What is the membrane potential? Use the mV form of the Nernst equation.
b. The intracellular [Ca+2] is also 100mM, and [Ca+2]extracellular=10mM. What is the membrane potential?
c. The Cl- anion concentration is 100mM inside the cell, and 10mM outside. What is the membrane potential?
d. You should have gotten three different values for a-c. Comment on the factor of the Nernst equation that accounts for these changes.
3) The cell can act as a capacitor. The voltage of a capacitor can be calculated from the following formula:
?? = Q/??
A particular cell has a membrane potential of -75 mV and a typical cell has a capacitance of 1 μF/cm2.
a. What is the total charge Q of such a cell?
b. Potassium (and any positive anion for that matter) ions will leave the cell to set up the cell membrane potential. If a K+ ion has a charge of +1.6×10-19 (the elementary charge), how many ions leave the cell?
c. A cell with a diameter of 50μm has a volume of 2.6×10-12 L and contains roughly 1.04×10-12 moles of K+ ions. Calculate the percent change in the concentration of K+ in the cell after the number of ions you found in part b leave the cell to set up the cell membrane potential.
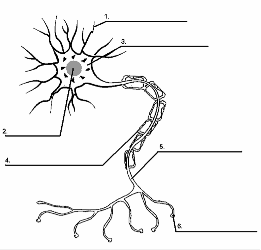
Label the following parts of a neuron