Reference no: EM13176543
Listed below are eight technical accounting terms introduced in this chapter:
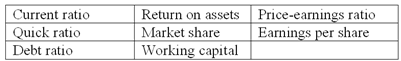
Each of the following statements may (or may not) describe one of these technical terms. For each statement, indicate the term described, or answer "None" if the statement does not correctly describe any of the terms.
(a) The percentage of total assets financed by creditors.
(b) A measure of the effectiveness with which management utilizes a company's resources, regardless of how those resources are financed.
(c) A company's percentage share of total dollar sales within its industry.
(d) Current assets less current liabilities.
(e) A measure reflecting investors' expectations of future profitability.
(f) A measure of short-term solvency often used when a company has large inventories that cannot be quickly converted into cash.
(g) A ratio that helps individual stockholders relate the net income of a large corporation to their equity investment.
|
How many grams of co2 gas are produced
: Tums calcium carbonate reactswith stomach acid ( HCI) according to the reaction below. One tablet of tums contains 500.0 mg CaCO3. If one tablet of tums is addedto 20.0 mL 0.100M HCI, how many grams of CO2 gas are produced?
|
|
Calculate the uncertainty in the position of an electron
: Calculate the uncertainty (in nm) in the position of an electron with the velocity of 2.00x103 m/s (the velocity is the same as in the preceding problem) and the uncertainty in the velocity is 1%. (2 significant figures are plenty).
|
|
What is the gdp growth rate
: nGDP is $5 trillion in Yr 1 and $5.4 trillion one year later. What is the GDP growth rate? B) If rGDP in Yr 1 was also $5 trillion what can you say about Yr1? C) If rGDP grew 5% between Yr. 1 and Yr. 2 what did GDP prices do?
|
|
Discuss in detail the healthcare applications of chemical
: Discuss in detail the healthcare applications of chemical reactions. Which chemical reactions would you consider to be of importance to healthcare professionals? Why? In which situations are these chemical reactions useful to healthcare profession..
|
|
Net income of a large corporation
: A measure of the effectiveness with which management utilizes a company's resources, regardless of how those resources are financed.
|
|
What mass of cacl is needed to make cacl
: What mass of CaCl is needed to make 350 mL of a 0.233M CaCl solution?
|
|
Find the number of months required tp pay off the balance
: For the same loan described under Q3, the individual decides that instead of selling the house after the 71st payment, to keep it and shorten the pay off period by increasing the montly payment by $150 each month. What is the number of months re..
|
|
What is the total pressure in the flask
: A 1.20 gram sample of dry ice is added to a 755mL flask containing nitrogen gas at a temperature of 25.0 degrees Celsius and a pressure of 725 mmHg. The dry ice is allowed to sublime and the mixture is allowed to return to 25.0 degrees Celsius. wh..
|
|
What is the charge on magnesium iodide
: What is the charge on magnesium iodide? What does the charge on the magnesium ion have to do with the number of valence electrons that an atom of magnesium has?
|