Reference no: EM13378423
Mechanical Behavior of Materials: Celebration of Learning 02 20 June 2014
This is a take-home celebration of learning. The deadline for this celebration is Tuesday 24 June 2014 at 18:00. Best of success to you all!
I. PHASE DIAGRAMS and MICROSTRUCTURE
I.A. By using the Iron-Carbon (Fe-C) phase diagram provided herein, answer the following questions: Sketch the microstructure of alloys A, B, and C in the following temperature ranges when they are cooled from 1000oC very slowly (equilibrium cooling). Identify all phases in the microstructures.
Comment on the mechanical properties of the resultant alloy and its constituent phases.
(i) For alloy A: T=900oC, TA>T>727oC and T<727oC,
(ii) For alloy B: T=726oC, T= 727oC and T <727oC
(iii) For alloy C: TC>T>727oC, T=727oC and T<727oC,
(iv) Would you chose an alloy in the Fe-C system with a carbon content greater or smaller than 0.77 wt% C if you needed a steel with moderate strength and good ductility? Justify your answer by taking microstructural considerations into account.
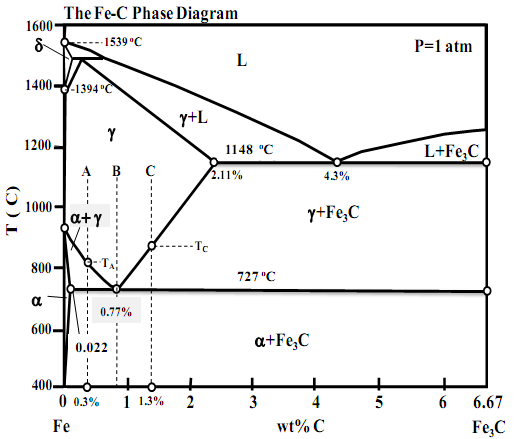
I.B. By using the Iron-Carbon (Fe-C) phase diagram provided herein, answer the following questions. You may mark your answers on the provided phase diagram. Show all you workings and give clear but short explanations in your bluebook.
(i) Identify all phases marked on the phase diagram. What are the reactions below 1200oC, write the reactions, including the temperature at which they occur and the % of carbon of each phase participating in the reaction.
(ii) What is the melting point of pure Fe? What is the melting temperature of Fe containing 4.3% carbon? What is the temperature at which d-Fe transforms to g-Fe?
(iii) Trace the crystallization path of alloys A, B, and C on the phase diagram. You may re-sketch the relevant portion of the phase diagram to mark the crystallization path of each alloy clearly if you are inclined to do so.
(iv) Calculate the phase composition of alloys A, B, and C at T=728oC and T=726oC.
(v) What are the pre-eutectoid phases in alloys A and B? Compute the % of pre-eutectoid phases present in alloys A and B at 726oC.
II. PHASE TRANSFORMATIONS Part I
II.A. (i) What is heterogeneous nucleation and why does it take place? Briefly, compare and contrast homogenous and heterogeneous nucleation.
(ii) What is the number of iron atoms found in a nucleus of critical size computed in (i). The lattice parameter of iron at the temperature of interest is 0.292 nm and its crystal structure is BCC with atoms per unit cell. (1 nm = 1 x 10-9m)
II. B. For the solidification of iron, calculate the critical radius r* and the activation free energy ΔG* if nucleation is homogeneous for a supercooling of 300 K. Values for the enthalpy of solidification and surface free energy are -1.85 × 109J/m3 and 0.204 J/m2, respectively. Sketch the variation of the surface, bulk and total energy with nucleus size and mark on it the r*and ΔG*. What does r*and ΔG* represent?
How does r*and ΔG* of a cube-shaped nucleus compare to a spherical nucleus?
III. PHASE TRANSFORMATIONS Part II
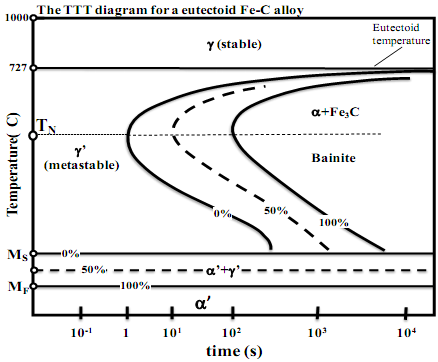
III.A. By using the time-temperature-transformation (TTT) diagram for a steel of eutectoid composition (Fe-C alloy) given below, answer the following questions. Sketch the cooling path(s) on the TTT diagram provided below in answering each question and label them clearly.
(i) What is meant by the so-called critical cooling rate? What happens if this eutectoid steel is quenched from 1000oC at a cooling rate that is faster than the critical cooling rate? Trace the cooling path on the diagram. What is the crystal structure before and after the quenching?
(ii) What happens to this eutectoid steel if it is first quenched to a temperature 727oC > T > TN and then isothermally held for a very long
time (t >104seconds) at that temperature, followed by slow cooling to room temperature? What is the microstructure immediately after quenching (t ≤ 1 sec), and after the isothermal hold (t > 104sec)?
III.B. By using the time-temperature-transformation (TTT) diagram for a steel of eutectoid composition (Fe-C alloy) given below, answer the following questions. Sketch the cooling path(s) on the TTT diagram provided below in answering each question and label them clearly.
(i) What do the temperatures Ms and Mf designate on the TTT diagram? What happens when this eutectoid steel is first quenched to Ms > T > Mf and then isothermally held for a very long time, followed by slow cooling to room temperature? What phases are present at room temperature? (ii) What happens to this eutectoid steel if is first quenched to a temperature Ms < T < TN and then isothermally held up to the 50% transformation curve, followed by quenching to T < Mf. (iii) What is the resultant phase or phases present in this eutectoid steel after the following heat treatment cycle: First quenched to Ms < T <MF at a cooling rate faster than the critical cooling rate and held for t > 104 sec, followed by reheating to T > 727oC and held for a long time, and then quenched to Ms < T < TN at a rate faster than the critical
cooling rate and held for t > 104 seconds and then quench to room temperature.
IV. PHASE TRANSFORMATIONS Part III and MICROSTRUCTURE
IV.A. (i) The kinetics of a solid state phase transformation in an engineering alloy is known to obey the Avrami equation given by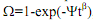 , where Ω is the fraction transformed, ψ and β are time-independent coefficients, and t is the time. The rate (R) of such transformations is given by R= (t0.5)-1
, where Ω is the fraction transformed, ψ and β are time-independent coefficients, and t is the time. The rate (R) of such transformations is given by R= (t0.5)-1
where t0.5 is the time needed for the transformation to be half-way complete. If the fraction transformed (Ω) is 0.45 after 200 minutes, determined the rate of this phase transformation. For this problem β = 2.5.
IV.B. (i) Why does the nucleation rate curve is bell-shaped? (ii) What is the eutectoid phase transformation in the Fe-C system? What kind of a phase transformation is it? At what temperature does it occur, what phases are involved, what are their compositions? Sketch the room temperature microstructure of a Fe-C alloy with 0.3 wt% C when it is very slowly cooled from 900oC
(iii) What is the martensitic phase transformation? How does it differ from the eutectoid (pearlitic) phase transformations? Compare and contrast the microstructure of the resultant phases. How are the crystal structures of the high temperature phase and the product phase related for the martensitic phase transformation. What is the tempering treatment typically applied to martensitic steels. (iv) Briefly, discuss by defining, comparing and contrasting gray and white cast irons.