Reference no: EM13897346
Guideline
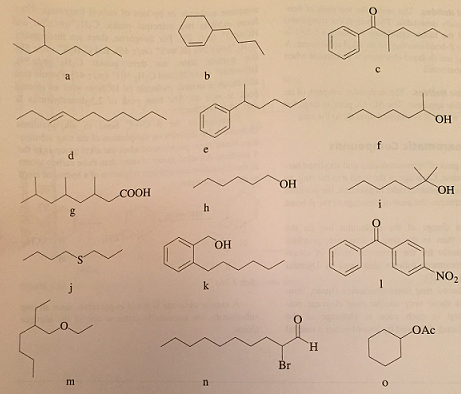
1. The relative intensity of the molecular ion peak is greatest fro the straight chain compound and decreases as the degree of branching increase.
2. The relative intensity of the molecular ion peak usually decreases with increasing molecular weight in a homologous series. Fatty esters appear to be an exception.
3. Cleavage is favored at alkyl-substituted carbon atoms; the more substituted, the more likely is cleavage. This is a consequence of the increased stability of a tertiary carbocation over a secondary, which in turn is more stable than a primary. Generally, the largest substituent at a branch is eliminated most readily as a radical, presumably because a long-chain radical can achieve some stability by delocalization of the lone electron.
4. Double bonds, cyclic structures, and especially aromatic (or heteroaromatic) rings stabilize the molecular ion and thus increase the probability of its appearance.
5. Double bonds favor allylic cleavage and give the resonance-stabilized allylic carbocation. This rule does not hold for simple alkenes because of the ready migration of the double bond, but it does hold for cycloalkanes.
6. Saturated rings tend to lose alkyl side chains at the alpha bond. This is merely a special case of branching. The positive charge tends to stay with the ring fragment. Unsaturated rings can undergo a retro-Diels-Alder reaction.
7. In alkyl-substituted aromatic compounds, cleavage is very probable at the bond beta to the ring, giving the resonance-stabilized benzyl ion, or more likely, the tropylium ion.
8. The C-C bonds next to a heteroatom are frequently cleaved, leaving the charge on the fragment containing the heteroatom whose nonbonding electrons provide resonance stabilization.
9. Cleavage is often associated with elimination of small, stable, neutral molecules, such as carbon monoxide, alkenes, water, ammonia, hydrogen sulfide, hydrogen cyanide, mercaptans, ketones, or alcohols, often with rearrangement.
|
Prince hamlet discovery about father death
: One of the major themes of the play might be described as "appearances vs. reality." In other words, the young prince Hamlet's discovery about his father's death has shaken the foundations of his being.
|
|
The most effective way for an employer to respond
: Of the following, the most effective way for an employer to respond to an employee's claim of retaliation would be:
|
|
Entry highlights several themes
: This entry highlights several themes that can be found in Milton's Doctrine of Discipline and Divorce. Love and happiness are at the heart of this literary masterpiece, and Milton isn't shy about expressing his views. For him, free will should be ..
|
|
Which of the following is a neutral requirement
: Which of the following is a neutral requirement that is likely to result in adverse impact?
|
|
Mass fragmentation patterns for a few compounds
: Mass fragmentation patterns for a few compounds
|
|
Three employees working for the same company
: Three employees working for the same company were found to have stolen company cargo. Two of the employees were white, and one was black. The two white employees were fired, but not the black employee. If the white employees sue, the court will mo..
|
|
What are airs? how do they work?
: What are AIRS? How do they work? Why is Banc One using them so extensively?
|
|
A cover letter for my legal research and writing class
: A cover letter for my legal research and writing class. The assignment I have to submit is a cover letter attached to a resume like i am applying for a position with the district attorney's office. I am not sure what to write or how long it should be..
|
|
The purpose of business is to make money
: Many would probably say that the purpose of business is to make money. Indeed, many people invest in business in order to make money. But, is that the sole purpose of business? While businesses do have a responsibility to make money, do they also h..
|