Reference no: EM131013295 , Length:
Please can you explain the answers to the questions in detail
Mass Spectrometry Coursework
1. Deduce what the major fragmentation process would be for the three compounds shown below. Identify which fragments are most likely to observed under EIMS, and calculate their m/z values.
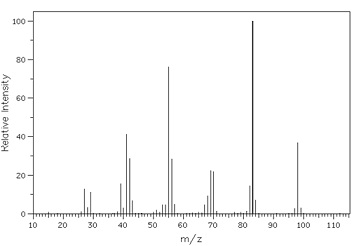
2. Below is shown the EIMS of methylcyclohexane. Locate the following information on it.
(i) The molecular ion
(ii) The base peak
(iii) The ion created by loss of a C3H7 radical from the molecular ion.
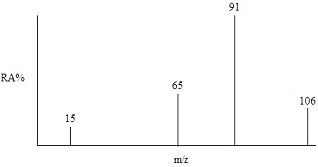
3. Below is shown a simulated EIMS of a compound with formula C8H10. Explain the origin of peaks at
(i) m/z = 91
(ii) m/z = 65
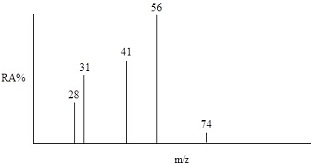
4. The simulated EIMS spectrum of an alcohol is shown below. The signal at m/z = 74 can be assumed to be the molecular ion. Identify the alcohol and suggest identities for the other ions in the spectrum.
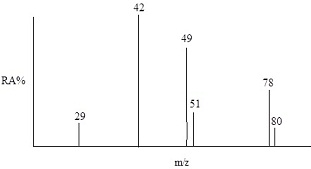
5. The simulated EIMS spectrum shown below is that of an alkyl halide. What is the molecular formula of this compound? Identify the structures of the all the ions detected.
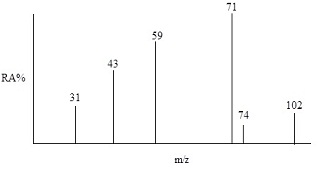
6. Identify the compound with molecular formula C5H10O2 shown in the simulated EIMS spectrum below.
7. The EIMS spectra of 3-methyl-2-pentanone and 4-methyl-2-pentanone can be told apart from theMcLafferty rearrangements which are observable. Explain.
|
What price would the investor receive a margin call
: Suppose that an investor sells 400 shares short at $50 per share. The initial margin is 50% and the maintenance margin is 30%. After 125 days, the investor purchases the shares for $40 and closes the short position. At what price would the investor r..
|
|
What is their role in an integrative global strategy
: How can managers assess the potential relative competitive position of their firm in order to decide on new strategic directions? Discuss the relative advantages of globalization versus regionalization/localization.
|
|
What is the return on equity for firm
: Firm A and Firm B have debt-total asset ratios of 35% and 25% and returns on total assets of 9% and 13%, respectively. What is the return on equity for Firm A and Firm B? (Do not round intermediate calculations. Enter your answers as a percent rounde..
|
|
Title insurance policy protecting-equitable title
: John sells his house to Mabel by giving a deed to Mabel. In the purchase agreement John was to buy a title insurance policy protecting Mabel's title. What kind of title policy did John buy Mabel? Coral sells her house to Sky using a land sale contrac..
|
|
Identify the structures of the all the ions detected
: The simulated EIMS spectrum shown below is that of an alkyl halide. What is the molecular formula of this compound? Identify the structures of the all the ions detected.
|
|
What knowledge skills abilities and traits you prioritize
: You are on the search committee for an art museum looking for a chief executive. What knowledge, skills, abilities, and traits would you prioritize
|
|
Bought a bond with an annual coupon rate
: Suppose you bought a bond with an annual coupon rate of 5.2 percent one year ago for $920. The bond sells for $970 today. Assuming a $1,000 face value, what was your total dollar return on this investment over the past year? If the inflation rate las..
|
|
Choose a company in the social media industry
: Choose a company in the social media industry or a chain in the fast-food industry. In small groups, conduct a multilevel environmental analysis, describing the major variables involved, the relative impact of specific threats and opportunities, an..
|
|
Retail margins on the product
: Horatio Alger has just become product manager for Brand X. Brand X is a consumer product with a retail price of $1.00. Retail margins on the product are 33%, while wholesalers take a 12% margin. Brand X and its direct competitors sell a total of 20 m..
|