Reference no: EM131189628
1. You have been asked to do a metabolism (energy) analysis of a person. How would you define the system for this purpose? What type of system is this?
2. You are trying to understand how a reciprocating air compressor (piston-cylinder device) works. What system would you use? What type of a system is this?
3. How might you define a system to study the depletion of ozone in the upper layers of the earth's atmosphere?
4. How would you define a system to determine the rate at which an automobile adds carbon dioxide to the atmosphere?
5. How would you define a system to determine the temperature rise created in a lake when a portion of its water is used to cool a nearby electrical power plant?
6. Is the weight of a system an extensive or intensive property?
7. The specific weight, g, of a system is defined as the weight per unit volume (note that this definition violates the normal definition for specific properties). Is the specific weight an extensive or intensive system property?
8. Is the number of moles of a substance contained in a system an extensive or intensive system property?
9. The molar specific volume of a system is defined as the ratio of the volume of a system to the number of moles of substance contained in the system. Is this an extensive or intensive property?
10. How would you describe the state of the air in the atmosphere? What kind of a process does this air undergo from the cool morning to a warm afternoon?
11. How would you describe the state of the water in a bathtub? How would you describe the process the water undergoes as it cools?
12. Is gc necessary in the SI unit system? If it is required, what are its units and magnitude?
13. A man weighs 180 lbf at a location where g = 32.10 ft/s2 . Determine his weight on the moon where g = 5.4 ft/s2 .
14. If the mass of an object is 10 lbm, what is its weight at a location where g = 32.0 ft/s2 ? (9.95 lbf)
15. A lunar exploration module weighs 4000N at a location where g = 9.8 m/s2 . Determine the weight of this module in newtons when it is on the moon where g = 1.64 m/s2 .
16. What is the weight (in newtons) of an object with a mass of 200 kg at a location where g = 9.6 m/s2 (1920 N)
17. The pressure in a compressed air storge tank is 1500 kPa. What is this pressure in (a) kN and m units, (b) kg, m, and s units, and (c) kg, km, and s units?
18. Determine the molecular weight of methane (CH4) and propane (C3H8) in kg/kmol units. (16.04 kg/kg-mol, 44.10 kg/kg-mol)
19. What is the molecular weight of R-12 (CCl2F2) in lbm/lbm-mol units?
20. A container is filled with 1 kg of a fluid whose specific volume is 0.001 m3 /kg and 2 kg of a fluid whose specific volume is 0.008 m3 /kg. What is the volume of this container (in m3 ) and the total weight (in newtons) of its contents at a location where g = 9.6 m/s2 ? (0.017m3 , 28.8N)
21. A mixture is 70% by volume liquid water whose density is 62.5 lbm/ft3 and another fluid whose density is 50.0 lbm/ft3 . What is the specific weight (in lbf/ft3 ) of this mixture at a location where g = 31.9 ft/s2 ?
22. One liter of an incompressible liquid whose specific volume is 0.0003 m3 /kg is mixed with 2 liters of another incompressible liquid whose specific volume is 0.00023 m3 /kg. What is the density of the resulting mixture in kg/m3 ? (4010 kg/m3 )
23. A container is filled with 1 kg of a fluid whose specific volume is 0.001 m3 /kg and 2 kg of a fluid whose specific volume is 0.008 m3 /kg. What is the volume of this container and the total weight of its contents at a location where g = 9.6 m/s2 ? (0.017 m3 , 28.8 N)
24. A mixture is 70% by volume liquid water whose density is 62.4 lbm/ft3 and another fluid whose density is 50.0 lbm/ft3 . What is the specific weight of this mixture at a location where g = 31.9 ft/s2 ?
25. One liter of a liquid whose specific volume is 0.003 m3 /kg is mixed with 2 liters of a liquid whose specific volume is 0.00023 m3 /kg in a container whose volume is 3 liters. What is the density of the resulting mixture? (3009 kg/m3 )
26. One pound-mass of a gas whose density is 0.001 lbm/ft3 is mixed with 2 lbm of a gas whose density is 0.002 lbm/ft3 such that the pressure and temperature of the gases do not change. Determine the resultant mixture's volume and specific volume.
27. One pound-mass of a gas whose density is 0.001 lbm/ft3 is mixed with 2 lbm of another gas whose density is 0.002 lbm/ft3 in such a way that the pressure and temperature do not change. Determine the resultant mixture's volume in cubic feet and its specific volume in ft3 /lbm.
28. The absolute pressure in a compressed air tank is 200 kPa. What is this pressure in psia?
29. The absolute pressure of the helium inside a child's balloon is 1000 mm Hg. What is this pressure in kPa? (133.3 kPa)
30. If the pressure inside the balloon of Problem 29 is 1500 mm Hg, what is the pressure in psia? (29.00 psia)
31. The water pressure in a home is 60 psi. What is the elevation of the water/air interface in the water tower serving this home?
32. The diameters of the pistons shown in Figure 1 are D1 = 3 in and D2 = 2 in. Determine the pressure in chamber 3 in psia when the other pressures are P1 = 150 psia and P2 = 200 psia. (110 psia)
33. The force generated by a spring is given by F = kx where k is the spring constant and x is the deflection of the spring. In Figure 2, this spring has a spring constant of 200 lbf/in and has been compressed 2 in. The piston diameters are D1 = 5 in and D2 = 2 in. What is P2 when P1 = 100 psia and P3 = 20 psia?
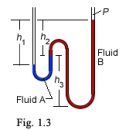
34. What is the absolute pressure applied to the left-hand column of the manometer shown in Figure 3 when h1 = 17 cm, h2 = 15 cm, h3 = 30 cm, γA = 100 kN/m3 , γB = 80 kN/m3 , and P = 90 kPa? (90Pa)
35. What is the absolute pressure applied to the left-hand column of the manometer shown in Figure 3 when h1 = 17 cm, h2 = 15 cm, h3 = 30 cm, γA = 10 kN/m3 , γB = 20 kN/m3 , and P = 745 mmHg?
36. Steam enters a heat exchanger at 300 K. What is the temperature of this steam in degrees Fahrenheit? (80 o F)
37. The temperature of the lubricating oil in an automobile engine is measured as 150 o F. What is the oil temperature in degrees Celsius?
38. Heated air is at 150 o C. What is the temperature of this air in degrees Fahrenheit? (302 o F)
39. What is the temperature of the heated air in Problem 1.38 in degrees Rankine?