Reference no: EM1379677
Thermometry is the science of temperature measurement. For a thermometer to work, we infer the temperature from the change in a physical property (termed the thermometric property). The system might be a platinum wire in which the property is resistance, a thermocouple (voltage) or a fixed gas volume (pressure).
The common type of thermometer is termed the liquid-in-glass type. For this type, the system is a fixed mass of liquid and the property is the volume of the liquid. For a thermometer to be effective the thermometric property should vary linearly with temperature over the required temperature range.
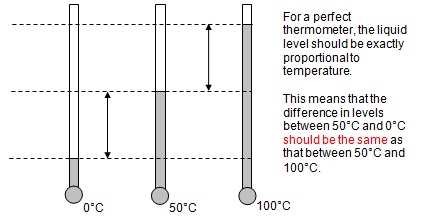
(a) The first part is a test of how to deal with algebra. Consider that we have an object of mass m, volume V and density r. The coefficient of thermal expansivity (a) is defined as:

This means that a can be specified in terms of density data, which is often more useful.
(b) The data below are the densities for a number of potential thermometric materials. These are termed 1, 2 and 3. You should recognise that liquid 1 is water?
|
T (°C)
|
r1 (kg/m3)
|
r2 (kg/m3)
|
r3 (kg/m3)
|
|
0
|
1000
|
808
|
13595
|
|
50
|
988
|
763
|
13473
|
|
100
|
958
|
711
|
13352
|
Using the information in the above table to show how the specific volume of each liquid varies with temperature. You should plot a graph for each liquid and use this graph to estimate the exact value of a at 50°C for each liquid.
Why might you find it better to plot the data for liquids 1 and 2 on a single graph, and that for liquid 3 on a separate graph?
Compare your calculated values with any useful values you can find from the literature.
What might limit the accuracy of your calculated values of a?
(c) Can you justify which is the best choice of thermometric fluid for a glass-liquid thermometer? This is best achieved by starting with a consideration of how you would expect the liquid to expand through the tube.
(d) Can you list three problems with using glass/liquid thermometers to measure temperature?
(e) For a large number of liquids, it can be shown that the variation of specific volume (v) (at constant pressure) with temperature can be fitted with a second-order polynomial:
V = aT2+bT +c
For an ideal thermometric liquid, what conclusions can you make regarding the values of the parameters a, b and c?
(f) A researcher suggests, as a low-cost measure, using a water-in-glass thermometer to measure temperatures as low as 1°C. Describe the "strange" behaviour of such a thermometer between the temperatures of 1°C and 4°C.
Note: you will need to make reference to a source of literature.