Reference no: EM13126659
Using the following graph to answer the questions below
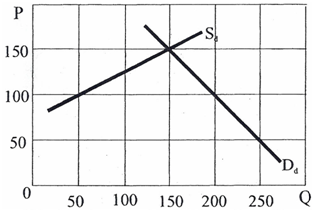
a. What would be the price of steel in the United States if a policy of "self-sufficiency" were established and no imports were allowed? How many tons of steel would be produced?
Price of steel: ______________________
Tons of steel: ______________________
b. If the world price of steel were $100 per ton and the United States adopted a free-trade policy, identify on the graph and state below how much steel would be produced in the United States, and how much would be imported.
Steel produced in US: ______________________
Steel imported: ______________________
c. Show on the graph and determine the dollar magnitudes of the different effects of a 20 percent tariff on steel imports.
Total consumer surplus loss: ______________________
Redistributive effect: ______________________
Protective effect: ______________________
Revenue effect: ______________________
Consumption effect: ______________________
|
What is the density of the metal
: The volume of a sphere is . A sphere of metal has a radius of 1.56 cm. It has a mass of 147.9 grams. What is the density of this metal?
|
|
Determining rate of change problem
: A car leaves Lima at noon traveling due West at 65 mph. At 2 PM, a car 300 miles south of Lima heads towards Lima at 70 mph. How quickly is the distance between the cars changing at 4 PM?
|
|
Explain what is the theoretical yield of vanadium
: What is the theoretical yield of vanadium that can be produced by the reaction of 40.0 g of V2O5 with 40.0 g of calcium based on the following chemical reaction.
|
|
What mass of calcium nitride form
: When 56.0 g calcium and 34.7 g nitrogen gas undergo a reaction that has a 88.0% yield, what mass of calcium nitride forms?
|
|
How many tons of steel would be produced
: What would be the price of steel in the United States if a policy of "self-sufficiency" were established and no imports were allowed? How many tons of steel would be produced?
|
|
Define important information about heat of reaction
: Important information about Heat of Reaction, The combustion of butane produces heat according to the equation 2C4H10(g) + 13O2(g) --> 8CO2(g) + 10H2O(l) deltaHrxn= -5314kJ
|
|
Amount of accrued interest payable
: The amount of accrued interest payable that should be shown on the December 31, 1998 balance sheet is ?
|
|
Illustrate what total amount should be credited additional
: The market value of the common stock at the date of the conversion was $30 per share. Illustrate what total amount should be credited to additional paid-in capital from common stock as a result of the conversion of the preferred stock into common..
|
|
Prepare ensinada purchases budgets for wax
: Ensnada Mfg. Co, rpoduces candles that contain four raw materials: wax, dye, scented oil and a wick. Each candle usues eight ounces of wax and one ounce of die, which Ensinada purchase for $0.15 and $0.02 per ounce, respepctively.
|