Reference no: EM131220734
1. Use the balanced equation to answer the question.
2 C2H6(g) + 5 O2(g)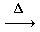 4 CO(g) + 6 H2O(g)
4 CO(g) + 6 H2O(g)
How many moles of O2 are needed to react completely with 1.8 moles of C2H6?
2. Enter your answer in the provided box.
Use the balanced equation to answer the question.
2 C2H6(g) + 5 O2(g) 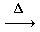 4 CO(g) + 6 H2O(g
4 CO(g) + 6 H2O(g
How many moles of H2O are formed from 0.10 moles of C2H6?
3. Enter your answer in the provided box.
Use the balanced equation to answer each question.
2 C2H6(g) + 5 O2(g) 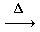 4 CO(g) + 6 H2O(g
4 CO(g) + 6 H2O(g
How many moles of C2H6 are needed to form 9.2 moles of CO?
4. Be sure to answer all parts.
Using the balanced equation for the combustion of acetylene, answer the following questions.
2 H-C≡C-H + 5 O2 →4 CO2 + 2 H2O
a. How many moles of O2 are needed to react completely with 7.40 mol of C2H2?
b. How many moles of C2H2 are needed to form 0.60 mol of CO2?
5.) Be sure to answer all parts.
Using the balanced equation for the combustion of acetylene, answer the following questions.
2 H-C ≡ C-H + 5 O2 → 4 CO2 + 2 H2O
a. How many moles of CO2 are formed from 9.0 mol of C2H2?
b. How many moles of H2O are formed from 0.13 mol of C2H2?
|
Program flowchart and corresponding pseudcode
: The Fibonacci series is defined to be the following numbers: 0,1,2,3,5.8,... where each number is the sum of the previous two numbers. Construct a program flowchart and corresponding pseudcode that will compute and output the first number greater ..
|
|
What needs to be done to create database
: Assume you have a customer who would like to track inventory including vendor (where the product comes from) and customer (who the product is sold to). What needs to be done to create this database?
|
|
Determining the middle frequency
: The resulting output from the modulation process is known as the middle frequency (MF) signal. True or false?
|
|
Examine and discuss whether permissive reporting
: Examine and discuss whether permissive reporting (i.e., personal or professional judgment regarding reporting) or mandatory reporting yields better results. Why or why not? When responding to this question, be sure to reference the lecture content..
|
|
How many moles of oxygen are needed to react completely
: Use the balanced equation to answer the question. How many moles of O2 are needed to react completely with 1.8 moles of C2H6?
|
|
Stakeholders and user groups of information systems
: ACCT6001 Accounting Information Systems Apply technical knowledge and skills in creating information for the workplace using spreadsheets and relational databases and communicate with IT professionals, stakeholders and user groups of information syst..
|
|
Comment on the given statement
: Comment on the statement: "People care about real interest rates, not nominal rates. Therefore, money demand should depend on the difference between the real rates on money and bonds, not the nominal rate on bonds."
|
|
What are the four levels of transactions defined in ibm’s dr
: What are the four levels of transactions defined in IBM's DRDA? Compare and contrast these four levels. Give examples to illustrate your answer.
|
|
How given development affect money demand and interest rate
: It becomes quick and inexpensive to sell bonds. In the liquidity preference theory, how does this development affect money demand and the interest rate?
|