Reference no: EM13897989
1) The surface shown of the irregularly shaped piece of copper below has an area of 0.27 m2 at 27°C.
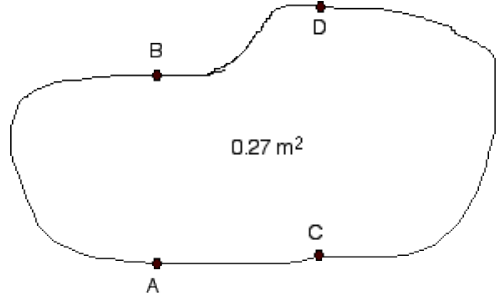
a) Determine its area when heated to 98°C. Recall the formula derived in class for the linear expansion of areas. It applies here even though the object is not rectangular.
b) At 27°C point B is 0.3 m from point A along a straight line and point D is 0.35 m from point C along a straight line. How far are these points from each other at 98°C?
c) If the object is to maintain the same shape (no matter how irregular) when heated how must the quantities ΔLAB/LAB and ΔLCD/LCD compare at various temperatures? The numerator in each case is the change in length and the denominator the initial length. Explain your
reasoning.
2) Diagrammed below is a bicycle air pump made of metal. The tube on the side is closed so no air escapes and the plunger can move up or down without letting air in or out.
a) Imagine that someone in their garage pushed down the plunger and then held it in place. In terms of the equilibrium thermodynamics described by the ideal gas law (and assuming the air in the pump can be treated as an ideal gas) which quantities have changed, which have not, over the course of this process? For those quantities that have changed, describe how they have changed. Explain your reasoning.
b) How would your answer to part a change (if at all) if the pump were perfectly insulated and the same process occurred?
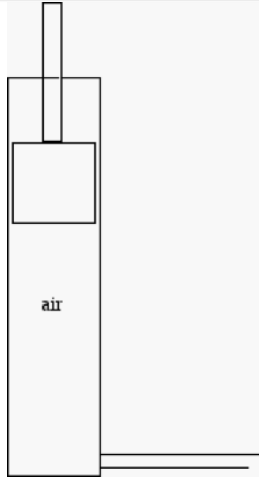
c) If left alone in the garage the plunger settles at a particular position. It's June and the garage is in Ohio. The uninsulated pump is then placed in a ice bath so that it's mostly surrounded by ice (lots of ice). Only the top of the plunger is uncovered and exposed to the air in the room. In terms of the equilibrium thermodynamics described by the ideal gas law what will happen? Which quantities will change? Which will not? For those that change, how will they change?
3) Two sealed metal drums are connected by a pipe of negligible volume . A valve in the pipe which is currently closed isolates one drum from the other. The larger drum, which is filled with gas,has a volume of 2L1 and the smaller drum, which is currently empty (a vacuum) has a volume of 1 L. The gas pressure (absolute) in the larger drum is 1.4 atm and both drums are in a room whose temperature is 25°C.
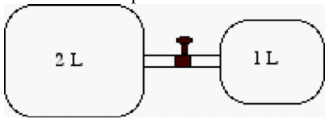
a) How many individual gas particles (either atoms or molecules) are in the filled drum? The valve is now opened. Gas flows until hydrostatic equilibrium is reached: the pressure is the same in each drum.
b) What will be the temperature of the gas at equilibrium? What volume will it occupy? Use the ideal gas law to determine the equilibrium pressure.
c) How many gas particles are in each drum at equilibrium?
d) How are pressure and volume related for this process? The system under consideration is the total number of gas particles initially in the larger drum. Briefly explain.
4) There is a direct relationship between the temperature of a substance and the average kinetic energy of the atoms (or molecules) in the sample. The relationship is: Kavg = 3/2kBT, where kB is the Boltzmann constant and T the Kelvin temperature (kB = 1.38 x 10-23 J/K). With this in mind consider the behavior of a metal container filled with 5.6 x 1023 Nitrogen molecules at 23°C.
a) Determine the kinetic energy of an "average" molecule in the container.
b) Determine the total kinetic energy of the 5.6 x 1023 molecules. If the gas is ideal, this is the internal energy of the gas as there is no potential energy, and we are assuming only translational degrees of freedom, that is, none of the internal energy is associated with rotation or vibration of the molecules.
c) Consider each of the processes described in question 2 (a,b and c) and the process that occurs after the valve is open in question 3. Use the definition of the internal energy of a gas stated in part b of this question to determine if the internal energy of the gas in each case has increased, decreased or remained the same. Explain your reasoning.