Reference no: EM132956052
Part 1:
1. This study focuses on the characteristics of the abundant plasma protein referred to as histidine- proline-rich glycoprotein (HPRG). HPRG is so named because it has a very high content of histidine (13 mol %). The human HPRG contains a pentapeptide GHHPH sequence repeated in tandem twelve times. The authors of this study hypothesized that its high histidine content might allow HPRG to play a role in regulating local pH in the blood. The local pH in blood may drop a half a pH unit during lactic acidosis or even a full pH unit in hypoxia or ischemia. In the case presented here, the binding of HPRG to glycosaminoglycans was investigated. Glycosaminoglycans are anionic polysaccharides that are the major component of the ground substance that forms the matrix of the extracellular spaces of the connective tissue in blood vessel walls. In this study, the binding of HPRG to the glycosaminoglycan heparin was measured. Based on their results, the investigators propose a model which describes how binding of HPRG to glycosaminoglycans may allow HPRG to regulate local blood pH.
Binding studies were carried out in which heparin was immobilized on the surface of cuvettes. The pH dependence of HPRG binding to heparin was investigated and the results are shown in Figure 1 below:
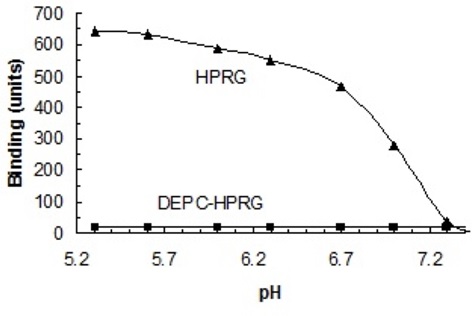
Figure 1. The pH-dependence of HPRG binding to unmodified heparin and heparin modified with DEPC
a. How is the binding of HPRG to heparin dependent on pH? Give structural reasons for the binding dependence. The structure of heparin is shown in Figure 2 below. NOTE: Figure 1 above also serves as a titration curve for the His residues in HPRG-what is the pKR for these residues? Explain.
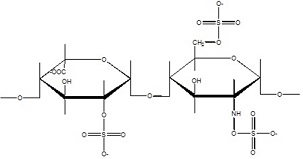
Figure 2. Repeating disaccharide unit of heparin
b. The same binding studies were carried out in which HPRG was reacted with diethylpyrocarbonate (DEPC), a compound that specifically reacts with histidine residues. The reaction is shown in Figure 3 below. Explain the results.
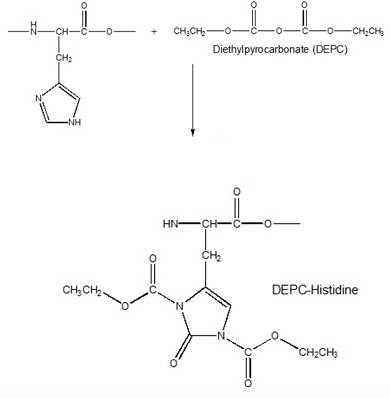
Figure 3. Reaction of histidine side chains with diethylpyrocarbonate
Part 2:
2. The effect of transition metals on the binding of HPRG was investigated next. The ability of increasing concentrations of Cu2+ and Zn2+ to promote HPRG binding to heparin at pH = 7.3 was measured. The results are shown in Figure 4 below. In addition, the binding of HPRG to heparin in the presence of these ions was compared at various pH's. Figure 5 below shows the comparison of binding of various concentrations of zinc ions at pH values ranging from 6.0 to
7.4. What is your interpretation of these results?
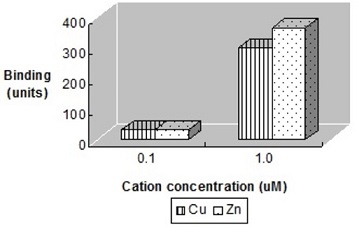
Figure 4. Binding of HPRG to heparin in the presence of copper and zinc ions
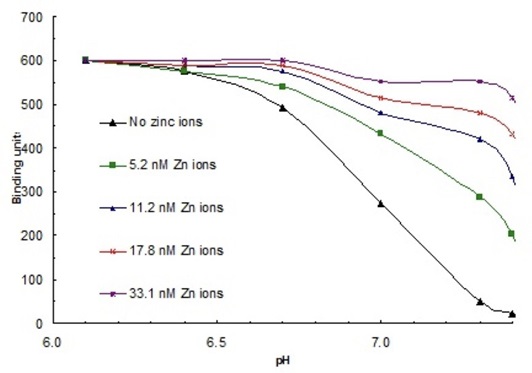
Figure 5. Binding of HPRG to heparin in the presence of increasing concentrations of zinc ions, at pH values 6.0-7.4.
Part 3:
3. Other plasma proteins have been studied for their ability to bind to glycosaminoglycans. One such protein is kininogen, which is a lysine-rich protein. Like HPRG, kininogen is able to bind to glycosaminoglycans, but this binding is far less sensitive to small fluctuations in physiological pH.
a. Why does kininogen bind to glycosaminoglycans easily?
b. Why is the binding of kininogen less sensitive to physiological pH changes?
Part 4:
4. The image below shows the structure of serotonin. Answer the questions below based on the structure of serotonin.
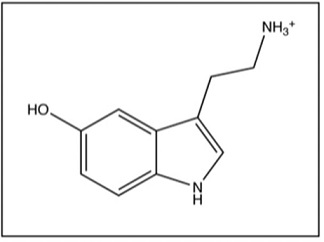
a. From which amino acid is serotonin derived?
b. What are the structural differences between serotonin and this amino acid (your answer to question 4 (a) above)?
c. What is the function of serotonin?
d. Which illness can be caused by reduced levels of serotonin in the brain?