Reference no: EM131245711
Question 1) What is the difference between closed and isolated systems?
Question 2) Is it true that thermodynamic equilibrium is reached very rapidly (a few seconds or less) in any system? Justify your answer or provide examples that support it.
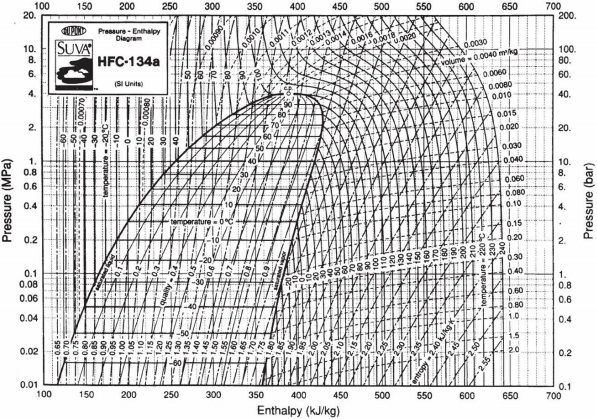
Question 3) A continuous flow of 1 kg/s of refrigerant HFC-134a at a pressure of 4 MPa and temperature of 120oC enters a valve, operating at steady-state, from which it leaves at 0.1 MPa and 40oC. What is the heat transfer rate in the valve?
Question 4) 22.8 kg of n-octane (C8H18, molar mass=114 g/mol) and the stoichiometric amount of oxygen are fed to a closed, isothermal reactor (700 Kelvin) of constant pressure (10 bar) and variable volume that initially contains no other chemicals. The reaction stops when all n-octane is consumed (final state).
a) Find the number of moles of oxygen, carbon dioxide, and water in the final state.
b) Assume the mixture in the final state is an ideal gas. Find its volume.
Question 5) A continuous ideal gas stream with flow rate equal to 20 moles/s at 900 K and 10 bar enters an adiabatic reversible turbine operating at steady-state, from which it exits at 2.5 bar. The molar heat capacity at constant pressure of this gas is equal to 30 J/(mol.K).
a) Starting from the general form of the mass, energy, and entropy balances, cancel out what is applicable to obtain working expressions for the situation described. Neglect changes in kinetic and potential energies.
b) Find the rate of entropy generation in the turbine.
c) Find the gas temperature after the turbine.
d) Find the power obtained in the turbine.
Question 6) An external wall that is adiabatic, rigid, and impermeable isolates a composite system from the surroundings. An internal wall separates the two subsystems A and B that form the composite system. Subsystem A contains ideal gas "a" whose molar heat capacity at constant pressure is equal to 21 J/(mol.K). Subsystem B contains ideal gas "b" whose molar heat capacity at constant pressure is equal to 29 J/(mol.K). In the initial state, the internal wall separating two subsystems A and B is adiabatic, rigid, and impermeable, and each subsystem has volume equal to 1 m3 and pressure equal to 5 bar. The initial temperatures in subsystems A and B are equal to 300 K and 600 K, respectively.
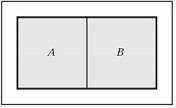
a) Find the number of moles present in each subsystem.
b) Assuming the internal wall becomes diathermal, rigid, and impermeable, find the temperature and pressure in each subsystem at equilibrium.
c) Assuming the internal wall becomes diathermal, moveable, and impermeable, find the temperature and pressure in each subsystem at equilibrium.
|
Why might it be difficult to get out of this kind of trap
: Why might it be difficult to get out of this kind of trap? Why is the government sometimes a part of the problem of coordination failure rather than the solution? Does this make the problem hopeless? What could be done in this case?
|
|
Which ones would be most difficult to escape
: Do you think developing countries can escape all of the traps described in this chapter? Which ones would be most difficult to escape? How could the developed world be of assistance in these cases? Could developed countries do more?
|
|
Create a policy that would benefit your organization
: If there any circumstances that might allow temporary exception to the policy, such as during an emergency, define them here. If there is anyone with the authority to temporarily waive the policy, they should be identified by job title. This secti..
|
|
Calculate and provide the annual sales revenues and costs
: Calculate and provide the annual sales revenues and costs. - Why is it important to include inflation when estimating cash flows?
|
|
Find the number of moles of oxygen
: CHEN 623 - Applications of Thermodynamics to Chemical Engineering Find the number of moles of oxygen, carbon dioxide, and water in the final state and Assume the mixture in the final state is an ideal gas. Find its volume.
|
|
Differences between formal and nonformal education
: What reasons would you give for the rather sizable school dropout rates in developing countries? What might be done to lower these rates?. What are the differences between formal and nonformal education? Give some examples of each.
|
|
Prepare end of year balance sheets and income statements
: CONS2010 Assignment. The government is inviting proposals from suitably qualified local contractors for the design, finance and construction of tourist facilities at sites including Valencia, Caroni, La Brea (Trinidad) and Castara (Tobago). Prepa..
|
|
Explain the concept of urban bias
: Why are some of the potential benefits of urbanization lost when congestion becomes substantial? What policies are likely to strengthen or weaken the opportunities to take advantage of the economic benefits of cities?
|
|
Analyze the means in which data moves within organization
: Suggest three (3) logical access control methods to restrict unauthorized entities from accessing sensitive information, and explain why you suggested each method. Analyze the means in which data moves within the organization and identify techniq..
|