Reference no: EM13720752
Question 1: Backward motion of the ratchet:
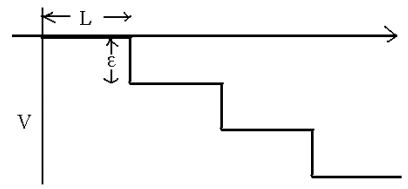
a) Find the average speed of a ratchet moving by steps of length L through a medium where its diffusion constant is D with no backward motion, as was done in the lectures.
b) Find the average forward speed if at each step a fraction of the ratchets step backward. Check the two limits ε = 0 and ε → ∞. Comment.
Question 2: Contributions to the capacitance of a lipid bilayer:
a) Estimate the capacitance of a lipid bilayer. Consider only the electrical insulating part of the bilayer, the lipid tails, as a layer of oil about 2 mu thick. The dielectric constant of oil is εoil / εo = 2.
b) The charge screening layers in the water (εwater = 80) on either side of the lipid bilayer also contribute to its capacitance. In physiological salt concentrations, these layers are each roughly 0.7 nm thick. Use the formula for capacitors in series from first year physics and your result in a) to estimate the contribution to the total capacitance from these layers.
Question 3: Discharging the battery: Imagine the resting membrane of the axon as a capacitor with specific capacitance C = 1 µF/cm2.
a) How much charge per unit area must pass through the membrane to discharge the capacitor (that is bring V from Vrest = -60mV to zero)?
b) Re-express your answer in a) by giving the surface area per excess proton charge needed to maintain Vrest = -60 mV. Then express it a third time, as the charge per unit length of the axon, taking the squid giant axon to be a cylinder of radius 0.5 nm.
c) As we shall see Wed. April 6, depolarization of the membrane (e.g. raising Vmem above 0 mV) is largely the result of the inflow of Na+ ions. Estimate the effect on the interior ion concentration of a charge transfer of the sort just described in a) and b) as follows. Again imagine the giant axon as a cylinder with salt solution, with ion concentrations given in the Table below:
|
II Ion
|
Valence z
|
Interior c2,i
(mM)
|
Relation
|
Exterior c1,i
(mM)
|
Nernst potential ViNernst
(mV)
|
|
KK+
Na+
|
+1
+1
|
400
50
|
>
<
|
20
440
|
-75
+59
|
|
Cl-
|
-1
|
52
|
<
|
560
|
-59
|
Find the total number of interior Na+ ions per unit length. Find the corresponding number if the interior Na+ concentration matched the exterior value. Subtract these two numbers and compare with the total number of sodium ions passing through the membrane as estimated in b).
d) Explain in the light of those numbers why an axon can continue to transmit many action potentials after the its ion pumps have been shut down.
Question 4: Vacuole equilibrium:
Here are some data for the marine alga Chaetomorpha. The extracellular fluid is seawater: the "plasmalemma" (outer cell membrane) separates the outside from the cytoplasm; a second membrane ("tonoplast membrane') separates the cytoplasm from an interior organelle, the vacuole.
|
Ion
|
Vacuole
(mM)
|
Cytoplasm
(mM)
|
Extracellular
(mM)
|
r*"' (plasmalemma)
(my)
|
remat (tonoplast)
(my)
|
|
K+
Na+
|
530
56
|
425
50
|
10
490
|
?
+57
|
-5.5
?
|
|
Cl-
|
620
|
30
|
573
|
-74
|
+76
|
a. The table gives some of the Nernst potentials across the two membranes. Fill in the missing ones.
b. The table does not list the charge density ρq,macro arising from impermeant macroions in the cytoplasm. What is - ρq,macro /e in mM?
c. The actual measured membrane potential difference across the tonoplast membrane is +76 mV. Suppose all the quoted numbers are accurate to about 2%. Which ion(s) must be actively pumped across the tonoplast membrane, and in which direction(s)?
Question 5: We analyzed the polymerization ratchet in the two limiting cases of diffusion-limited and reaction-limited polymerization. By comparing the time for a load, polystyrene sphere 1 µm in diameter, to diffuse a distance given by the actin monomer size, and the average time for an actin monomer to be added to the growing end of the filament, find the condition for the free actin monomer concentration that is necessary for the polymerization in the presence of the load to be reaction-limited. Compare this concentration with the critical concentration for actin filament growth.
|
Evaluate the effectiveness of chosen company statement
: For this discussion activity, you will research a company's of your choice and locate the company's most current mission statement. In order to evaluate the effectiveness of the chosen company's statement you will use the following criteria for ev..
|
|
Annual deposits into a retirement account
: You make $9,600 annual deposits into a retirement account that pays 9.8 percent interest compounded monthly. Required: How large will your account balance be in 31 years?
|
|
How can you synthesize this into the workplace
: For the average business leader who is not in a finance role how do risk, return and the cost of capital impact him or her? How can you synthesize this into the workplace?
|
|
What is the raroc of the loan
: What is the RAROC of the loan and under what circumstances should the bank make the loan?
|
|
Find the average speed of a ratchet
: Find the average speed of a ratchet moving by steps of length L through a medium where its diffusion constant is D with no backward motion, as was done in the lectures
|
|
Assignment on an operations plan for a company
: Create an operations plan for my company using SAMPLE Operations Plan Preparation Form. Extract appropriate information from the NAB Company portfolio, where applicable. Other required items in the template should be filled in using your personal ..
|
|
How did romes contact with the hellenistic world
: How did Rome's contact with the Hellenistic world affect Roman civilization in the second and third centuries B.C.E.? Provide specific examples of art, architecture, literature, religion, and philosophy.
|
|
What are major types of customers catherine serves
: What are the three major types of customers Catherine serves? How do they differ from one another? What do you think the order winners are for each group?
|
|
How sas used organizational strategy to drive hrm practices
: Consider how SAS used organizational strategy to drive HRM practices. What are they doing that is different than most others? How and why did SAS do what they did?
|