Reference no: EM131402118
Assignment -1
In nucleophilic substitution bimolecular(SN2) reactions of alkyl halides, the order of reactivity is: R-I>R-Br>R-Cl>R-F, where R is an alkyl group. Alkyl Iodides are around 105 to 106 times more reactive than alkyl fluorides.
Contrary to this, 1-halo-2,4-dinitrobenzenes react at almost the same rate, in nucleophilic aromatic substitution. What could be the possible reason for this difference in the reactivity of haloalkanes and substituted haloarenes?
Assignment -2
What is the pH of a buffer system consisting of 0.30 MNH3 and 0.35 MNH4Cl buffer. What is the change in pH after the addition of 20.0 mL of 0.050 M NaOH to 70.0 mL of this buffer solution?
(Kb of NH3 = 1.8 x10-5)
Assignment -3
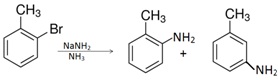
Aryl halides undergo nucleophilic substitution in presence of a strong base such as sodium amide in liq.ammonia. If o-bormotoluene is made to react with this base, o-toluidine and m-toludine are formed in equal amounts instesd of m-toluidine being the major prodict. How do you account for this fact?
Assignment -4
In an experiment, a 10 g piece of iron (specific heat = 0.45 J/g 0C) at 100 °C is dropped into 25 g of water (specific heat = 4.2 J/g 0C) at 27 degree C. Find temperature of the iron and water system at thermal equilibrium.
Assighment- 5
In an experiment, 25 mL of 0.10 M acetic acid is titrated with 0.10 sodium hydroxide solution. What is the pH of the solution after the addition of 25 mL of NaOH. (ionization constant of the conjugate base = 5.6x10-10)
|
Calculate the cost of capital of investing in a project
: Suppose the market risk is 6.2% and the risk free interest rate is 4.6% Calculate the cost of capital of investing in a project with a beta of 1.3
|
|
Standard deviation of the stock returns
: A stock produced returns of 11 percent, 19 percent, and 2 percent over three of the past four years, respectively. The arithmetic average for the past four years is 9 percent. What is the standard deviation of the stock's returns for the four-year..
|
|
Retirement account on your 66th birthday
: 1. You believe you will need $150,000 annually to live comfortably while retired. You plan on retiring when you are 65 and will begin withdrawing funds from your retirement account on your 66th birthday. If you expect to need 25 years of retirem..
|
|
What is the firm net float
: In addition, the firm generally receives an average of $140,484 a day in checks that are deposited immediately. Deposited funds are available in 1 days. What is the firm's net float?
|
|
Find temperature of the iron and water system
: In an experiment, 25 mL of 0.10 M acetic acid is titrated with 0.10 sodium hydroxide solution. What is the pH of the solution after the addition of 25 mL of NaOH. (ionization constant of the conjugate base = 5.6x10-10)
|
|
Disbursement float as of the end of the day
: As of this morning, your firm had a ledger balance of $3,656 with no outstanding deposits or checks. Today, your firm deposited 6 checks in the amount of $266 each and wrote 12 checks in the amount of $716 each. What is the amount of the disbursem..
|
|
Which course of action advances the common good
: Which course of action advances the common good? What moral rights do the affected parties have, and which course of action best respects those rights?
|
|
How many days are in the operating cycle
: ABC Corporation currently has an inventory turnover of 10.77, a payables turnover of 5.41, and a receivables turnover of 11.14. How many days are in the operating cycle?
|
|
Average accounts payable balance
: ABC Company has annual sales of $400,000 and cost of goods sold of $153,422. The accounts payable period is 59.91 days. What is the average accounts payable balance?
|