Reference no: EM131108440
1. Iron (II) sulphate, FeSO4, is easily oxidised to iron(III) sulphate.
(a) Calculate the percentage by mass of iron in iron(II) sulphate. ........................................ %
(b) An impure sample of iron(II) sulphate was analysed by titration. The sample was dissolved in 25.0 cm3 of dilute sulphuric acid and then titrated against 0.0400 mol/dm3 potassium dichromate(VI) solution.
19.0 cm3 of potassium dichromate(VI) solution was required to reach the end-point.
(i) Calculate the number of moles of potassium dichromate(VI) used in the titration......................................... moles
(ii) One mole of potassium dichromate(VI) reacts with six moles of iron(II) ions.Calculate the mass, in grams, of iron(II) ions in the sample analysed.mass of iron(II) ions........................................ g
2. A 0.24 g sample of magnesium ribbon is added to 5.0 cm3 of 2.0 mol/dm3 ethanoic acid.
Explain why this reaction forms the same volume of hydrogen but takes place much more slowly than the reaction of the same mass of magnesium with 5.0 cm3 of 2.0 mol/dm3 hydrochloric acid.
3. A 1.2 g sample of powdered brass was analysed by reaction with excess dilute sulphuric acid.
The zinc reacts as shown in the equation to form 0.072 dm3 of hydrogen measured at room temperature and pressure.
Zn + 2H+ → Zn2+ + H2
(i) Suggest why brass was used in a powdered rather than lump form.
(ii) Calculate the mass of zinc in the sample of brass.
(iii) Calculate the percentage of zinc in the sample of brass.
4. 2Mg + CO2 → 2MgO + C
(a) When 2 moles of magnesium react with one mole of carbon dioxide, 810 kJ of energy are released.
Calculate the energy released when 2.0 g of magnesium reacts completely with carbon dioxide.
5. a) 25.0 cm3 of an aqueous solution of calcium hydroxide is exactly neutralised by 18.0 cm3 of 0.040 mol/dm3 hydrochloric acid.
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
Calculate the concentration, in mol/dm3, of the aqueous calcium hydroxide.
6. Analysis of 10.0 g of carboxylic acid X shows that it contains 2.67 g carbon, 0.220 g hydrogen and 7.11 g oxygen.
(i) Deduce the empirical formula of X.
(ii) The relative molecular mass of X is 90. Deduce the molecular formula of X.
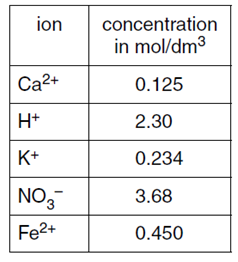
7. The table shows the concentration of different ions found in a sample of aqueous industrial waste.
8. Ethanol can also be manufactured from glucose, C6H12O6.
C6H12O6 →2CO2 + 2C2H5OH.
A solution containing 18 kg of glucose makes only 0.92 kg of ethanol.
Calculate the percentage yield of ethanol.