Reference no: EM131141739
Section A
Please answer the following questions and explain your reasoning
a) Carboxylic acids such as 1 (where X indicates an alkyl group) commonly undergo α-cleavage when subjected to electron impact mass spectrometry.
1) Give the mechanism for α-cleavage and predict which signals (quoted as either m/z values in amu, or as differences in mass between starting material and fragmentation product, in amu) you would observe during EI-MS?
2) Explain your reasoning?
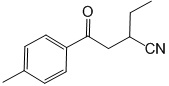
b) The molecule 2 may undergo a McLafferty rearrangement.
1) Draw a mechanism to show how this process operates for this molecule?
2) Identifying the fragments that result?
3) Indicating which fragments would be observed in EI-MS?
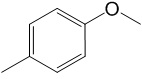
c) Predict the molecular ion mass and the identities of the TWO major fragment ions that might be observed for compound 3.
Section B
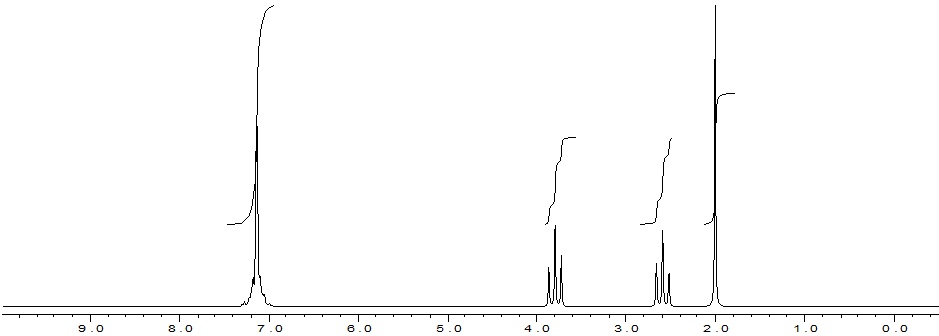
(a) The 1H NMR spectrum of compound C1(a) C10H12O2 (in CDCl3) is shown below.
1. Use this information to deduce the molecular structure for compound C1(a).
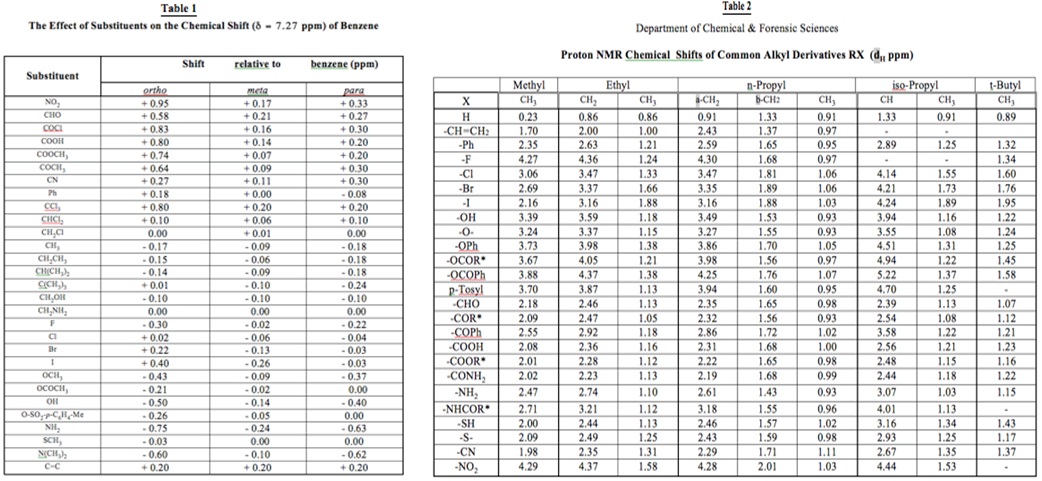
2. Show your reasoning throughout and confirm your answer (and the exclusion of alternative isomers) as far as possible by reference to the appropriate chemical shift tables provided below.
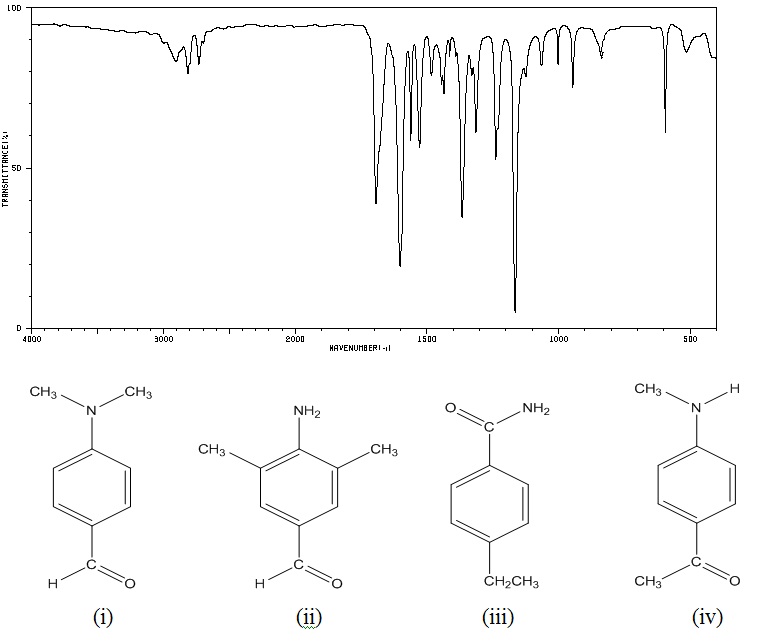
(b) The infrared spectrum (below) is that of one of the compounds (i) - (iv) shown underneath.
1) Deduce with explanation which compound is responsible for the spectrum?
2) Explain why the other structures do not produce the spectrum shown?
3) Assign the diagnostic and significant bands in the spectrum to specific vibrations in the molecule?