Reference no: EM133037847
CHT214 Formative Workshop
Question 1. Answer ALL parts a) - d).
Compound 1 is an anti-arrythmia drug that is obtained as a single enantiomer using the following one-pot, multi-enzyme mixture:
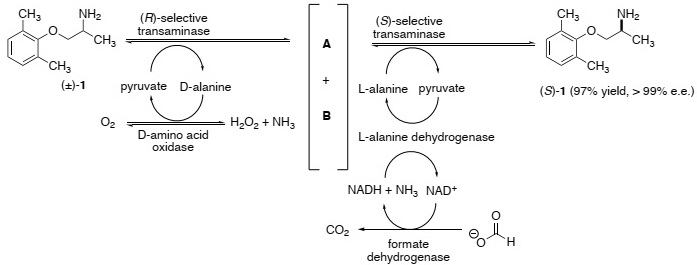
a) Draw structures for compounds A and B.
b) Which compound(s) is (are) consumed in this de-racemisation process?
c) Briefly explain why the enzyme formate dehydrogenase is added to the reaction mixture.
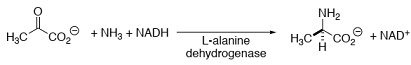
d) Write a curly arrow mechanism for the following reaction in the cascade that clearly shows the role of the co-factor NADH.
Question 2. Answer ALL parts a) - d).
Pictet-Spenglerases have been shown to catalyse reactions similar to the one shown below.
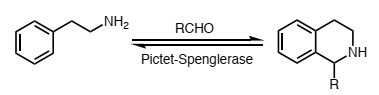
(a) Write a curly-arrow mechanism for this reaction.
(b) A product D (MW = 255; [α]D > 0°) when pyruvate and the hydroxybenzylamine C were incubated with a Pictet-Spenglerase and an (S)-selective transaminase. Propose a structure for D and briefly explain why a large excess of pyruvate was added to the reaction mixture.
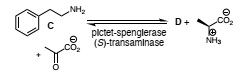
(c) The same (S)-transminase was used in the following enzyme cascade:
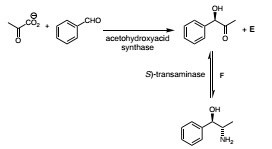
Draw the structure of the co-factor required by the enzyme acetohydroxyacid synthase and propose a structure for the product E.
(d) Propose a structure for the reagent F and briefly explain why it is added at the same concentration as the starting aldehyde even though the initial concentration of pyruvate is 100x lower.
|
What factors do you think that covid-19 effects
: -Given your knowledge of PESTLE, what factors do you think that COVID-19 (Coronavirus) effects? Explain your answer in detail.
|
|
Community relationship programs
: Discuss the current community relationship programs currently underway at your agency.
|
|
What is the materials price variance
: Seaside Company produces picture frames. During the year 190,000 picture frames were produced. What is the materials price variance
|
|
Define a technology accelerator
: Define a technology accelerator? Give an example of a company, either from good to great or from personal experience
|
|
Explain why the enzyme formate dehydrogenase
: Briefly explain why the enzyme formate dehydrogenase is added to the reaction mixture and Write a curly arrow mechanism for the following reaction
|
|
Create the stockholders equity section of the balance sheet
: Preferred stock, par value P100, 10 000 shares authorized Issued 5,000 shares P500,000. Create the Stockholders Equity section of the Balance Sheet
|
|
Current ehr system at independence medical center
: Based on the role you undertook in the media simulation, provide a brief analysis of the current EHR system at Independence Medical Center, as well as comment o
|
|
What is the cost of common equity
: What is the cost of common equity? Round your answer to two decimals place
|
|
How much were the noncash assets sold
: How much were the noncash assets sold for in order for A to receive the amount priority to her and an additional 7,500
|